��Ŀ����
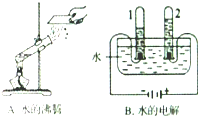
 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��С��ͬѧ������һ������ˮ������ͼ��ʾ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��С��ͬѧ������һ������ˮ������ͼ��ʾ����1����ȡһ�����ĺ�ˮ���������У��ȼ����������������ܽ⣬����һ��ʱ�䣬���뾻ˮ���й��ˣ���������������
������������ˮ���ɵĽ�״������ʵ�������ʹ���ʳ���
������������ˮ���ɵĽ�״������ʵ�������ʹ���ʳ���
����2���ڣ�1���й��˺��ˮ��֪��Ӳˮ������ˮ������
�������ˮ
�������ˮ
������������������
�������
�ķ���������ˮ��Ӳ�ȣ���3�����˺��ˮ�п��ܺ��н϶��ϸ���������������͵�ɱ��������������أ�K2FeO4����������Ԫ�صĻ��ϼ�Ϊ
+6��
+6��
����������1������ˮ�ľ��������������ý��з������
��2������Ӳˮ����ˮ�ļ��鷽��������ˮӲ�ȵķ������з������
��3�������ڻ��������������ϼ۴�����Ϊ�㣬��ϸ�����أ�K2FeO4���Ļ�ѧʽ���н���⣮
��2������Ӳˮ����ˮ�ļ��鷽��������ˮӲ�ȵķ������з������
��3�������ڻ��������������ϼ۴�����Ϊ�㣬��ϸ�����أ�K2FeO4���Ļ�ѧʽ���н���⣮
����⣺��1������ˮʱ�����������ã�������������ˮ���ɵĽ�״������ʵ�������ʹ���ʳ�����
��2��������˺��ˮ��֪��Ӳˮ������ˮ���ɼ������ˮ��������ĭ�϶������ˮ�����ٵ���Ӳˮ�������п��ü�����еķ���������ˮ��Ӳ�ȣ�
��3����Ԫ����+1�ۣ���Ԫ����-2�ۣ�����Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+1����2+x+��-2����4=0����x=+6�ۣ�
�ʴ�Ϊ����1��������������ˮ���ɵĽ�״������ʵ�������ʹ���ʳ�������2���������ˮ��������У���3��+6�ۣ�
��2��������˺��ˮ��֪��Ӳˮ������ˮ���ɼ������ˮ��������ĭ�϶������ˮ�����ٵ���Ӳˮ�������п��ü�����еķ���������ˮ��Ӳ�ȣ�
��3����Ԫ����+1�ۣ���Ԫ����-2�ۣ�����Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+1����2+x+��-2����4=0����x=+6�ۣ�
�ʴ�Ϊ����1��������������ˮ���ɵĽ�״������ʵ�������ʹ���ʳ�������2���������ˮ��������У���3��+6�ۣ�
�����������ѶȲ�������ˮ�ľ�����Ӳˮ����ˮ�ļ��鷽��������ˮӲ�ȵķ��������û��ϼ۵�ԭ�����ָ��Ԫ�صĻ��ϼ۵ķ���������ȷ����⣮

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
�����Ŀ
 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ������ˮ��Դ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ������ˮ��Դ��
 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��