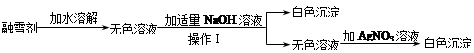��Ŀ����
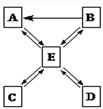
��4�֣�A��B��C��DΪ���г��������ʣ�A��B֮�䷢���Ļ�ѧ��Ӧ�ɱ�ʾΪ����A+B��C+D����
��1����CΪ�Ȼ��ƣ�DΪ���������A��Һ�м��������ܲ�����������AΪ ����A��B����Һǡ����ȫ��Ӧ����Ӧ����Һ��PH 7�������������=������
��2����A��B��Ϊ��ɫ��ĩ��A��B��Ӧʱ�ɹ۲쵽��ɫ��ĩ��Ϊ��ɫ��ͬʱ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��A��B��Ӧ�Ļ�ѧ����ʽΪ ��
��1����CΪ�Ȼ��ƣ�DΪ���������A��Һ�м��������ܲ�����������AΪ ����A��B����Һǡ����ȫ��Ӧ����Ӧ����Һ��PH 7�������������=������
��2����A��B��Ϊ��ɫ��ĩ��A��B��Ӧʱ�ɹ۲쵽��ɫ��ĩ��Ϊ��ɫ��ͬʱ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��A��B��Ӧ�Ļ�ѧ����ʽΪ ��
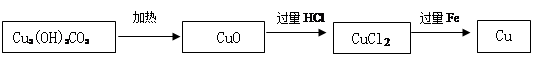
���ᡡ������������C+2CuO 2Cu+CO2��
2Cu+CO2��
 2Cu+CO2��
2Cu+CO2�������������1��A��Һ�м��������ܲ�����������ôA���������CΪ�Ȼ��ƣ���A�����ᡣ��ô�÷�ӦӦ���кͷ�Ӧ��D��ˮ��B�Ǽ��A��B����Һǡ����ȫ��Ӧ����Ӧ����Һ�����ԣ���Һ��PH=7��
��2����ɫ��ĩ��Ϊ��ɫ����ô��̼��ԭ����ͭ������ͭ�Ͷ�����̼��

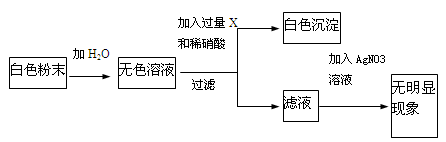
��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�
�����Ŀ