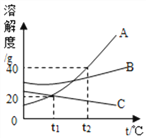
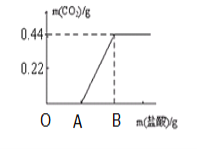
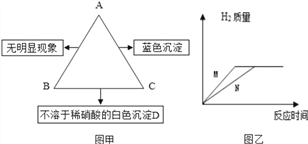
��Ŀ����
����Ŀ��ij���Ƽ�˵����IJ�����Ϣ��ͼ��ʾ������20Ƭ��ҩ���ƳɵĹ����ĩ��40gϡ������(�����ɷֲ������ᷴӦ)��ϡ����ƽ���ֳ�4�μ����ձ��У�ʵ�����ݼ��±���

ʵ����� | ����ϡ���������/g | �����ĩʣ������/g |
1 | 10 | 18.9 |
2 | 10 | 17.8 |
3 | 10 | 16.8 |
4 | 10 | m |
(1)��ƿ���Ƽ�����__________��(�����ʷ���)
(2)�����е�m=________��
(3)��ϡ�����Ũ��____________��(����ݻ�ѧ����ʽд�������ļ��㲽��)
(4)��ƿ���Ƽ�����̼���________g��(�������1λС��)
(5)������������ݣ�����ͼ�в�ȫͼ��
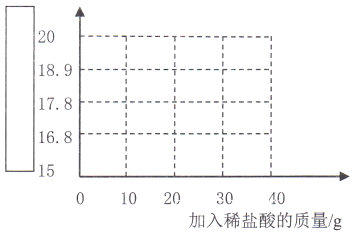 ___________
___________
���𰸡� ����� 16.8 8.03% 16.0 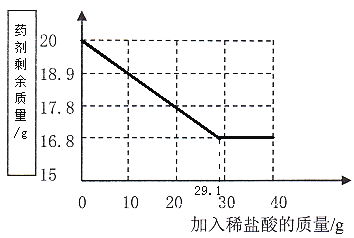
����������1�����Ƽ���Ч�ɷ���̼��ƻ����������ɷ֣����ڻ���
��2������ͼ����Կ�����ǰ���μ������ᣬ����ÿ�μ���1.1g���������μ������ᣬ����ÿ�μ���1.0g��˵��̼�������ȫ��Ӧ��������ʣ�࣬���Ե��Ĵμ������ᣬ�����ڷ�����Ӧ�������ĩʣ��������δ16.8g��m=16.8��
��3���⣺��ϡ�����Ũ��Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
10073
20g-19.8g10g��x
![]()
x=8.03%
��ϡ�����Ũ��Ϊ8.03%��
��4����ƿ���Ƽ�����̼���![]() ����20g-16.8g��=16.0g
����20g-16.8g��=16.0g
��5������ÿ����10gϡ����������������1.1g�����Թ������20g-16.8g =3.2gʱ������ϡ���������Ϊ![]() =29.1g�����Լ�������������������֮��Ĺ�ϵΪ��
=29.1g�����Լ�������������������֮��Ĺ�ϵΪ��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�