0 70323 70331 70337 70341 70347 70349 70353 70359 70361 70367 70373 70377 70379 70383 70389 70391 70397 70401 70403 70407 70409 70413 70415 70417 70418 70419 70421 70422 70423 70425 70427 70431 70433 70437 70439 70443 70449 70451 70457 70461 70463 70467 70473 70479 70481 70487 70491 70493 70499 70503 70509 70517 151629

 1.Through 1987 what was the ratio(比例)of the highest to the average wind speed for BM?
1.Through 1987 what was the ratio(比例)of the highest to the average wind speed for BM?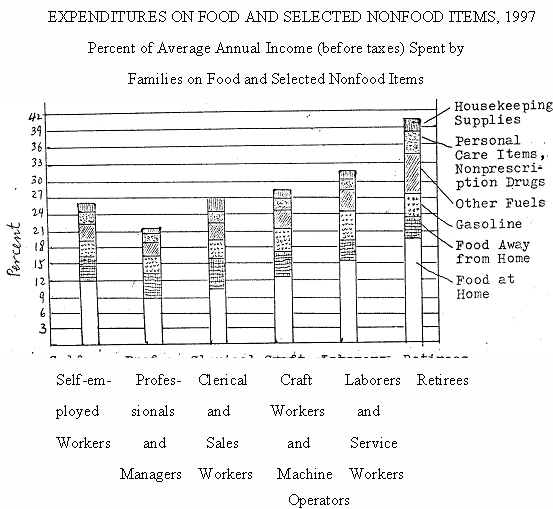
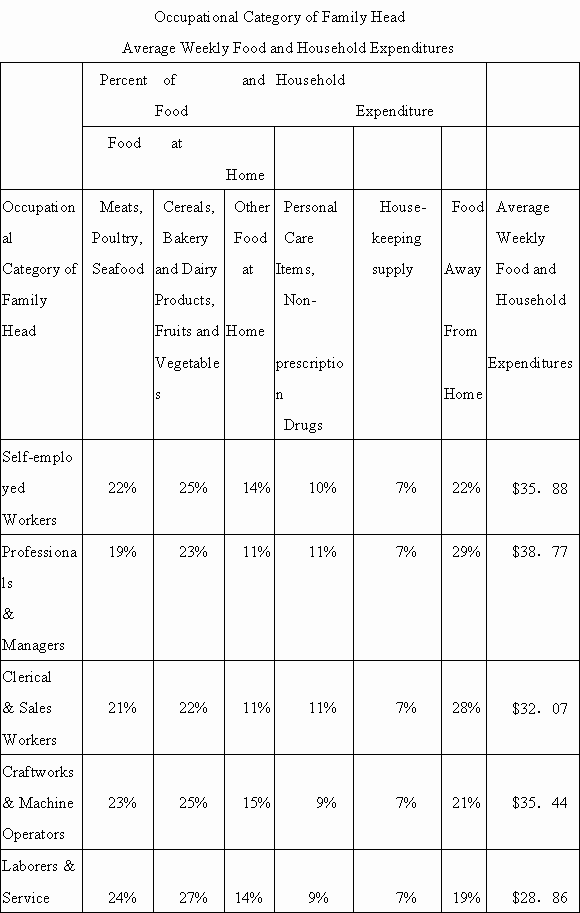 1.For which of the following categories was the percent of the average annual income (before taxes) spent on food at home the least?
1.For which of the following categories was the percent of the average annual income (before taxes) spent on food at home the least?

