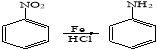��Ŀ����
����Ŀ��
����![]() �ϳ�
�ϳ� ��������ͼ��ʾ��
��������ͼ��ʾ��

��ش�����������
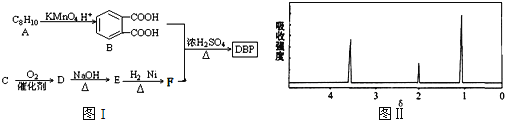
��l������A������Ϊ__________��A��B�ķ�Ӧ������__________��
��2����A�Ʊ�����ͪ��![]() ���Ļ�ѧ����ʽΪ________________��
���Ļ�ѧ����ʽΪ________________��
��3����F����G�Ļ�ѧ����ʽΪ________________��
��4��D��ͬ���칹���к�����Ԫ�����ܷ���������Ӧ����________�֡�
��5��д����������Ҫ���I��ͬ���칹��Ľṹ��ʽ_______��дһ�ּ��ɣ���֪ͬһ��̼ԭ���ϲ�������2���ǻ�����
�ٷ����廯���� �ڶ�Ԫ�� �۷�������5�ֻ�ѧ��������ԭ��
��6������������Ϣ����A�ͻ���ͪ��![]() ��Ϊԭ�ϣ������Լ���ѡ����д���ϳ�
��Ϊԭ�ϣ������Լ���ѡ����д���ϳ�![]() ������ͼ��
������ͼ��
___________
���𰸡� ���촼 ��ȥ��Ӧ 2![]() ��O2
��O2![]()
![]() ��2 H2O
��2 H2O ![]() ��3NaOH
��3NaOH![]()
![]() ��2NaBr��3H2O 5 ��2�֣�
��2NaBr��3H2O 5 ��2�֣� ![]() ��
�� 
![]()
��3�֣��������Լ��ĺϳɷֿ�дҲ��ȷ��
�����������������������Ҫ�����л���Ľṹ�����ʡ�
��l������A������Ϊ���촼��A��B���촼ת��Ϊ����ϩ����Ӧ��������ȥ��Ӧ��
��2����A�Ʊ�����ͪ��![]() ���������Ĵ�������Ӧ����ѧ����ʽΪ2
���������Ĵ�������Ӧ����ѧ����ʽΪ2![]() ��O2
��O2![]()
![]() ��2H2O ��
��2H2O ��
��3����F����G����±��������ȥ��Ӧ����ѧ����ʽΪ ![]() ��3NaOH
��3NaOH![]()
![]() ��2NaBr��3H2O ��
��2NaBr��3H2O ��
��4��D��ͬ���칹���к�����Ԫ�����ܷ���������Ӧ��Ҫ�������������̼ԭ�ӣ�����һ����ȩ����ʽ���ڣ���һ���Ǽ���ȩ���ͼ���������ͬһ��̼ԭ�ӣ�Ҳ���Դ����ڡ��䡢�Ե�λ�ã���������ȩ������ʽ���ڣ�����5�֡�
��5���Ա���Ϊ���������ӽṹ�Գƣ��γ�5����ԭ�ӵĽṹ��ʽ��Ϊ![]() ��
�� ��
��
��6��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�