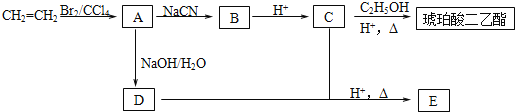
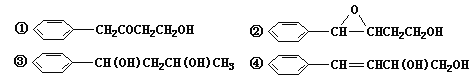��Ŀ����
����Ŀ������ȫ������Ϲ����к���ƻ����(MLA)�������ʽΪC4H6O5��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2(��״��)��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
(1)д���������ʵĽṹ��ʽ��A____________________��D____________________��
(2)ָ����Ӧ���ͣ���__________����__________��
(3)д��������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��__________________��
(4)д��E��Fת���Ļ�ѧ����ʽ________________________��
(5)����ת����ϵ�в�����������˳���ܷ�ߵ���_______(��ܡ����ܡ�)˵�����ɣ�_________________________��
(6)PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ___________________��
���𰸡�
(1)CH2BrCH=CHCH2Br��OHCCH2CHBrCHO��
(2)�ӳɣ�ȡ����
(3)
(4)HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O��
(5)���ܣ�����������B��̼̼˫��Ҳ��������
(6)![]() ��
��![]() ��
��
��������
���������ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2(��״��)��������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��Ӧ����1��-OH�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH(OH)COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ����(PMLA)����ṹΪ![]() ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH(OH)COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH(OH)COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
(1)������������֪��AΪCH2BrCH=CHCH2Br��DΪOHCCH2CHBrCHO���ʴ�Ϊ��CH2BrCH=CHCH2Br��OHCCH2CHBrCHO��
(2)��Ӧ����1��3-����ϩ���巢��1��4�ӳ�����BrCH2CH=CHCH2Br����Ӧ����BrCH2CH=CHCH2Br���������Ƶ�ˮ��Һ����ȡ����Ӧ����HOCH2CH=CHCH2OH���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
(3)������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�У�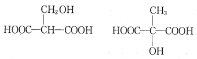 ���ʴ�Ϊ��
���ʴ�Ϊ��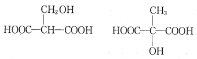 ��
��
(4)E��Fת���Ļ�ѧ����ʽΪ��HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O���ʴ�Ϊ��HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O��
(5)˳���ܵߵ�������������B��̼̼˫��Ҳ���������ʴ�Ϊ�����ܣ�����������B��̼̼˫��Ҳ��������
(6)PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ��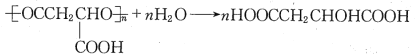 ���ʴ�Ϊ��
���ʴ�Ϊ��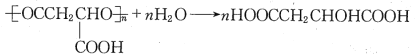 ��
��

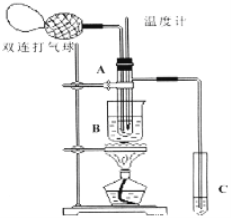
����Ŀ��ij��ȤС��ͬѧ��ʵ�����ü���l��������ŨH2SO4���廯�ƻ����ķ������Ʊ�1���嶡�飬���������ͼ��ʾ��ʵ��װ�������еļг�����û�л�������

��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���� ������װ���ж��õ��������ܣ�Aװ������ˮ�� ������ĸ���������룬Bװ������ˮ�� ������ĸ���������롣
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ�����CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� ��
��4��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
���� | �۵㣯�� | �е㣯�� |
1������ | ��89.5 | 117.3 |
1���嶡�� | ��112.4 | 101.6 |
���� | ��95.3 | 142.4 |
1����ϩ | ��185.3 | ��6.5 |
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶��� �ռ�������֡�