��Ŀ����
1�����\���������������ʵ����ϵ���У���ش����й������\��ʵ�������⣮��1���ڹ��ƹ�����Ȼ�������У���ĸ������Դ�Ǹ����ڹ�Ƥ�ϵ�Ұ���ͽ�ĸ��������װ���ڼ����֭��Ҫ����Լ$\frac{1}{3}$�Ŀռ䣬Ŀ���������ڽ�ĸ���������������п��ٷ�ֳ����ֹ�������в���������̼��ɷ���Һ������ƾ����ͺ�ת����ᷢ��ǰ�����ı��¶��⣬��������ķ���������ͨ����������
��2����ͨ��ĸ��ֱ�����õ��۵��������������˽�����ѿ�߸˾��Ħ�-����ø����ת���ĸ���У���ɸѡ�õ��˿ɸ�Ч���õ��۵Ĺ��̽�ĸ�����֣�

ͼһ�еĢڡ��۹������ظ����Σ�Ŀ���ǽ�һ��������÷ֽ��������ǿ�Ľ�ĸ����ijͬѧ���Թ��̢۵IJ���������һ��ƽ�徭������ľ���ֲ���ͼ����ʾ���Ʋ��ͬѧ����ʱ���ܵIJ���ʧ����Ϳ�������ȣ�
��3���Ʊ��̶�����ĸϸ��ʱҪ���ɽ�ĸ��������ܻ��õĺ���������Һ��ȴ�����£������ѻ�Ľ�ĸϸ�������г�ֽ��裬ʹ���Ͼ��ȣ���ת����ע�����У��Ժ㶨���ٶȻ����ؽ�ע�����е���Һ�μӵ����ƺõ��Ȼ�����Һ�У��۲���������γɣ�
���� ����ͼһ��ͼһ�ǽ�����ѿ�߸˾��Ħ�-����ø����ת���ĸ���о�ɸѡ�õ��˿ɸ�Ч���õ��۵Ĺ��̽�ĸ�����ֵĹ��̣�ͼ�Тٱ�ʾ�����������Ĺ������̣��ڡ��۹����ظ����ε�Ŀ���Ǵ�����÷ֽ��������ǿ�Ľ�ĸ����
����ͼ����ͼ����һ��ƽ�徭������ľ���ֲ����ɼ�����õ���ϡ��Ϳ��ƽ�巨����ͼ��Ⱥ��ֲ���Խϼ��У�
��� �⣺��1���ڹ��ƹ�����Ȼ�������У���ĸ������Դ�Ǹ����ڹ�Ƥ�ϵ�Ұ���ͽ�ĸ��������װ���ڼ����֭��Ҫ����Լ$\frac{1}{3}$�Ŀռ䣬�����ڽ�ĸ���������������п��ٷ�ֳ����ֹ�������в���������̼��ɷ���Һ����������������������˾ƾ����ͺ�ת����ᷢ��ǰ�����ı��¶��⣬����ͨ����������
��2�����������֪����ͨ��ĸ��ֱ�����õ��۵����������������̽�ĸ�����Ը�Ч���õ��ۣ�������ѿ�߸˾��Ħ�-����ø����ת���ĸ���У�ɸѡ�õ��˿ɸ�Ч���õ��۵Ĺ��̽�ĸ�����֣�ͼһ�еĢڡ��۹������ظ����Σ��ܹ���һ��ɸѡ������÷ֽ��������ǿ�Ľ�ĸ����ͼ����һ��ƽ�徭������ľ���ֲ����ɼ�����õ���ϡ��Ϳ��ƽ�巨����ͼ��Ⱥ��ֲ���Խϼ��У��Ʋ��ͬѧ����ʱ����Ϳ�������ȣ�
��3���Ʊ��̶�����ĸϸ��ʱҪ���ɽ�ĸ��������ܻ��õĺ���������Һ��ȴ�����£������ѻ�Ľ�ĸϸ�������г�ֽ��裬ʹ���Ͼ��ȣ���ת����ע�����У��Ժ㶨���ٶȻ����ؽ�ע�����е���Һ�μӵ����ƺõ��Ȼ�����Һ�У��۲���������γɣ�
�ʴ�Ϊ��
��1�������ڹ�Ƥ�ϵ�Ұ���ͽ�ĸ�� �����ڽ�ĸ���������������п��ٷ�ֳ����ֹ�������в���������̼��ɷ���Һ��� ͨ��������
��2����-����ø ɸѡ������÷ֽ��������ǿ�Ľ�ĸ�� Ϳ��������
��3���������� �Ȼ���
���� �����Թ��̾�Ϊ�زģ����ͼ���ۺϿ�����̡������ʵ�������������ƹ���������DNA����ȡ�ͼ������й�֪ʶ��Ҫ����ʶ�ǻ��̵Ĺ����������裻ʶ������Ľ��ַ������ܸ���ͼ���жϽ��ַ�����ͼ��������ֵ�ԭ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���Ǻ��� | B�� | �������� | C�� | ���� | D�� | ������ |
| A�� | ���߶����ڹ���ϸ������������ | |
| B�� | �������ߵĻ����Ǽܶ���̼�� | |
| C�� | �����ڵ����ʵ�����ϳ��о�����Ҫ���� | |
| D�� | ���ߵ����Ԫ���ж�����C��H��O��N��P |
| A�� | �����������������⣬���е������嶼��ϸ������ | |
| B�� | ��ϸ���ڷ�����Ľṹ������ϸ���ⳤʱ������������� | |
| C�� | ���ɲ�ͬ����ϸ���Ļ�ѧԪ�ص�����ͺ�����ͬ | |
| D�� | �������ܶ��������³´�л����������ֳ����������ϸ���н��� |
| ��Ŀ | H2O | NH4+ | K+ | Ca2+ | PO43- |
| ������ | 0% | 83% | 72% | 97% | 84% |
���������Ӷ����٣�˵��������Щ���Ӷ�������
�۸����������ӵ��������в��죬˵���������ӵ����վ���ѡ����
��Ca2+��K+���Լ��٣�˵��ˮ����ϸ��Ĥ������Ca2+�����������K+������࣮
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢۢ� | D�� | ����ȷ |
 ͼΪijϸ�������ṹģʽͼ������ͼʾ�ش���������
ͼΪijϸ�������ṹģʽͼ������ͼʾ�ش���������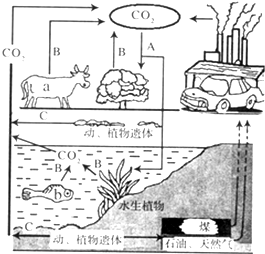 ������������ȫ�������ЧӦ����Щ����̨�籩�겻�ϣ���Щ��������Ӹɺ�����2009��籾�������Ϲ�����仯�����ϣ��й�������ŵ����2020�굥λGDP��̼�ŷ�������2005���½�40%--45%������̼���ѳ�Ϊÿһ���˵����Σ�������̼����ָ��������������ֱ�ӻ��ӽ������������ģ��Ӷ�����̼���ŷţ���ͼ��ij����ѭ������ʾ��ͼ�������������ݣ��ش����⣮
������������ȫ�������ЧӦ����Щ����̨�籩�겻�ϣ���Щ��������Ӹɺ�����2009��籾�������Ϲ�����仯�����ϣ��й�������ŵ����2020�굥λGDP��̼�ŷ�������2005���½�40%--45%������̼���ѳ�Ϊÿһ���˵����Σ�������̼����ָ��������������ֱ�ӻ��ӽ������������ģ��Ӷ�����̼���ŷţ���ͼ��ij����ѭ������ʾ��ͼ�������������ݣ��ش����⣮ �������������Ƿֽ��л���̵�ͼ�����£�
�������������Ƿֽ��л���̵�ͼ�����£�