��Ŀ����
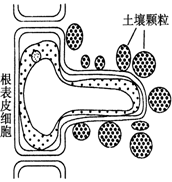
ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�
(1)�ò�λ�ı�Ƥϸ���빦������Ӧ�Ľṹ�ص���________________________��
________________________��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��________________________________����ʱ����������ˮ�ֺͿ���������
________________________________________________________________________��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��С�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺��ȡһ�ɾ����ز�Ƭ�����������1��__________________________����˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���__________��������__________��
ʵ����ۣ��ɴ�֤����
________________________________________________________________________��
(1)ϸ��������ͻ��ĸ�ë(��ϸ����������ͻ��)���д�Һ�ݡ�(2)ϸ��ʧˮ�������ʱڷ��롡��������ˮ�֣��������տ������ӡ�(3)1 mol/L���������Һ���ʱڷ��롡�Զ���ԭ��ϸ��ʧˮ�����ʱڷ���״̬ʱ���������տ�������
����

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�