��Ŀ����
��1������������Ϊ28%��KOHˮ��Һ�У�OH-������H2O������֮����_______��
��2����KCl��CaCl2����ɵ�ij������У�K+��Ca2+�����ʵ���֮��Ϊ2��1����û�����к�CaCl2����������Ϊ__________��KCl��CaCl2�����ʵ���֮��Ϊ__________����1 mol Cl-�ĸû�����������____________g��
��3���ڱ�״���£���CO��CO2��ɵĻ������13.44 L,����Ϊ20 g,�û�������У�̼��������ԭ�ӵ���Ŀ֮��Ϊ__________��
(1)5��4
(2)42.7% 2��1 86.7
(3)3��4
����:
��1���ֱ����KOH��H2O��������ת�������ʵ�����
��2�����������ʵ�����n��KCl����n��CaCl2��=2��1��ϸ������㡣
��3����CO��CO2�ֱ�Ϊx mol,y mol
(x+y)��22.4=13.44 ��
(28x+44y)=20 ��
����x+y��/(x+2y)=3��4��

��ϰ��ϵ�д�
�����Ŀ
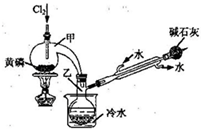 ��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�