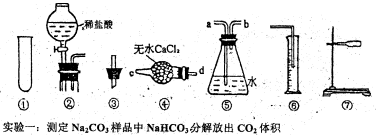
��Ŀ����
���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ���������������ѧ֪ʶ���ش��������⣺
�Ź�ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��Ư����Ч�ɷ�Ϊ�������ƣ� ��ƿװƯ�۾��ÿ����л��ϡ��״��ʧЧ�����û�ѧ����ʽ��ʾʧЧ��ԭ�� ��
��2��ij��ѧ��ȤС��Ϊ��̽��HClO��Ư���ԣ���������µ�ʵ�顣

a.ͨ��Cl2�Ӽ���ƿA��B������ɵó��Ľ����� �����з����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
b.Ϊ��ȷ����HClOʹʪ��ĺ�ɫ������ɫ������Ϊ��Ӧ���ӵ�ʵ���� ��
c.�ձ�����Һ����������� ���ձ�����������Ӧ�Ļ�ѧ����ʽΪ ��
d.Ϊ��֤ʵ�鰲ȫ��������ÿ����1.12L���ѻ���Ϊ��״���£�����������ͨ�룬��ʵ�����ʱ4���ӣ�С�ձ���ʢ��2 mol��L��1��NaOH��Һ���������ӦΪ mL��
mL��
�Ź�ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��Ư����Ч�ɷ�Ϊ�������ƣ� ��ƿװƯ�۾��ÿ����л��ϡ��״��ʧЧ�����û�ѧ����ʽ��ʾʧЧ��ԭ�� ��
��2��ij��ѧ��ȤС��Ϊ��̽��HClO��Ư���ԣ���������µ�ʵ�顣

a.ͨ��Cl2�Ӽ���ƿA��B������ɵó��Ľ����� �����з����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
b.Ϊ��ȷ����HClOʹʪ��ĺ�ɫ������ɫ������Ϊ��Ӧ���ӵ�ʵ���� ��
c.�ձ�����Һ����������� ���ձ�����������Ӧ�Ļ�ѧ����ʽΪ ��
d.Ϊ��֤ʵ�鰲ȫ��������ÿ����1.12L���ѻ���Ϊ��״���£�����������ͨ�룬��ʵ�����ʱ4���ӣ�С�ձ���ʢ��2 mol��L��1��NaOH��Һ���������ӦΪ
 mL��
mL��
��

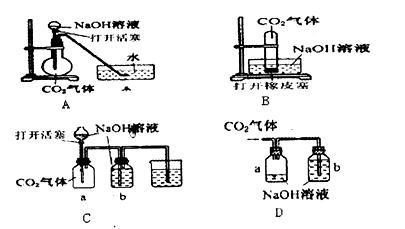
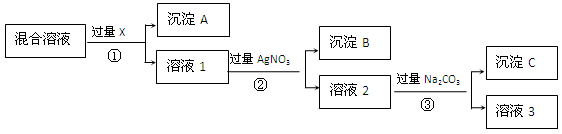
��ϰ��ϵ�д�
�����Ŀ

 ���У�Ȼ����ע��10 mL CCl4���Ǻò�������������������̨�ϣ��ȷֲ��ȡ�ϲ�Һ
���У�Ȼ����ע��10 mL CCl4���Ǻò�������������������̨�ϣ��ȷֲ��ȡ�ϲ�Һ
 ���¿̶ȡ�
���¿̶ȡ� Щʵ��
Щʵ�� ��������� ��
��������� �� 12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á�
12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á� 2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�
2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�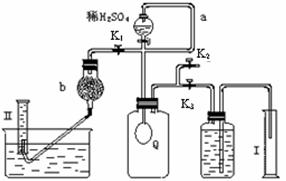
 ____________
____________ _____��
_____�� ����ͲҺ�����ˮ��Һ��
����ͲҺ�����ˮ��Һ��