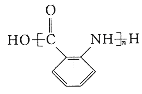
��Ŀ����
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ���������ʺ���������ȥ����A��������Ԫ����ɵ�������ˮ�����ʣ�����Ԫ�ص�������Ϊ7:4��B��ǿ�C��Ħ������Ϊ34g��mol-1�� F���������ɫҺ�壻��ɫ����G��ʹƷ����Һ��ɫ������I����Һ�еμ�KSCN��Һ����Һ����Ѫ��ɫ��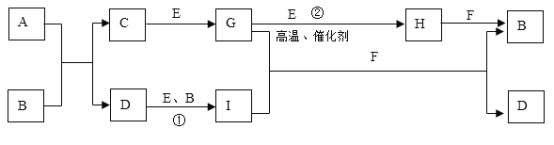
��ش��������⣺
��1��A�Ļ�ѧʽ____________________________��
��2��C�ĵ���ʽ____________________________��
��3����Ӧ�ٵ����ӷ���ʽ_______________________________________________________��
��4����Ӧ�ڵĻ�ѧ����ʽ_______________________________________________________��
���𰸡� FeS ![]() 4Fe2+ + O2 +4H+ ==4Fe3+ + 2H2O 2SO2 + O2
4Fe2+ + O2 +4H+ ==4Fe3+ + 2H2O 2SO2 + O2 ![]() 2SO3
2SO3
��������GΪ�����ҿ�ʹƷ����ɫ��GΪSO2������C��Ħ������Ϊ34g��mol-1��֪CΪH2S��EΪO2��HΪSO3��FΪH2O��BΪH2SO4������I����Һ�еμ�KSCN��Һ����Һ����Ѫ��ɫ��IΪFe2(SO4)3���Ӷ�DΪFeSO4������Ԫ���غ��֪A�к���S��FeԪ�ء�
��1��A�к���Fe��S����Ԫ�أ�ԭ�Ӹ�����Ϊ(7/56):(4/32)=1:1������A�Ļ�ѧʽΪFeS��
��2��CΪH2S������ʽΪ![]() ��
��
��3��D��E��B��I�ֱ�ΪFeSO4��O2��H2SO4��Fe2(SO4)3��Ӧ�����ӷ���ʽΪ��4Fe2+ + O2 +4H+ ==4Fe3+ + 2H2O��
��4����Ӧ����SO2��O2������������Ӧ����SO3����ѧ����ʽΪ��2SO2 + O2 ![]() 2SO3��
2SO3��

����Ŀ��ij����ѧϰС����ѧϰ��![]() ��
��![]() �ķ�Ӧ����Ϊ
�ķ�Ӧ����Ϊ![]() ��
��![]() Ӧ��Ҳ���Է�Ӧ�������������ͼװ��
Ӧ��Ҳ���Է�Ӧ�������������ͼװ��![]() �г�װ������ȥ��װ�õ�����������
�г�װ������ȥ��װ�õ�����������![]() ����ʵ�飬̽��
����ʵ�飬̽��![]() ��
��![]() ��Ӧ�IJ���밴Ҫ��ش��������⡣
��Ӧ�IJ���밴Ҫ��ش��������⡣
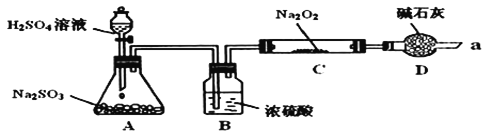
��![]() д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��______��
д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��______��
��![]() װ��D�����ã����˿��Է�ֹ�����е�
װ��D�����ã����˿��Է�ֹ�����е�![]() ��ˮ�����Ƚ���C����
��ˮ�����Ƚ���C����![]() ��Ӧ��������______________________________________________________��
��Ӧ��������______________________________________________________��
��![]() ��ͨ��������
��ͨ��������![]() ��
��![]() ��ַ�Ӧ�����Ƕ�C�й������������¼��裺
��ַ�Ӧ�����Ƕ�C�й������������¼��裺
����1��ֻ��![]() ��
��
����2��___________��
����3������![]() ������
������![]() ��
��
��1��������2�������![]() ��
��![]() ��Ӧ�Ļ�ѧ����ʽ��_________________��
��Ӧ�Ļ�ѧ����ʽ��_________________��
��2��ijͬѧ���������ʵ���һ��ȷ�ϲ���ijɷ֡�
ʵ�鲽�� | ���� |
| ����ȫ���ܽ� |
| ���� |
| ������ɫ���� |
����![]() �н�����������ͨ����������
�н�����������ͨ����������![]() ��Һ�У�������Ӧ�����ӷ���ʽΪ��______��ͨ������ʵ������ȷ������______������
��Һ�У�������Ӧ�����ӷ���ʽΪ��______��ͨ������ʵ������ȷ������______������![]() ѡ��1��2��
ѡ��1��2��![]()
����Ŀ��SO2�dz�������������ڻ����������������Ӧ�ù㷺��
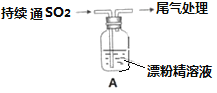
I��ͼ��ʵ������ȡSO2����֤SO2ijЩ���ʵ�װ��ͼ
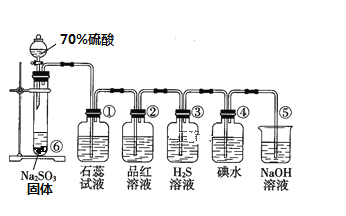
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________
��2������ʵ������Ϊ_____________________________________��֤��SO2��____��
��3���������ӷ�Ӧ����ʽΪ______________________
IIijѧ����SO2��Ư�۾��ķ�Ӧ����ʵ��̽����
���� | ���� |
ȡ4gƯ�۾����壬����100mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
���ˣ���Ư�۾���pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
| i.Һ���Ϸ����ְ��� ii.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ iii.�Ժ���������ɫ����������ɫ��ȥ |
��1��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������_________________
��2����ˮ�г���ͨ��SO2��δ�۲쵽�������Ʋ�����i�İ�����HClСҺ���γɣ���������ʵ�飺
a����ʪ��ĵ⻯�ص�����ֽ����������ޱ仯��
b�����ữ��AgNO3��Һ���������������ɫ������
��ʵ��a��Ŀ����_______________________________________________
����ʵ��a��b�����жϰ����к���HCl��������_______________________________________
��3����Aƿ�л������ˡ�ϴ�ӣ��õ�����X
�������X�м���ϡ���ᣬ�����Ա仯��ȡ�ϲ���Һ������BaCl2��Һ��������ɫ�����������X�к��е�������___________
�������ӷ���ʽ��������iii�л���ɫ��ȥ��ԭ�� ___________________________