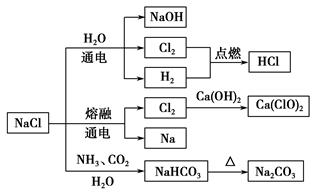
��Ŀ����
ˮ��������ˮ�ľ�����ɱ������������ȡ����г�����ɱ������������������������Ư�ۡ�����̿�ȡ���Ӿ���������ó���������̿����Ӿ�ؽ��������;���������˵������ȷ���� (����)��
| A������������̿����ˮ��ԭ����ͬ |
| B��������һ�ֳ��õ���������������������ˮҲ�����������ƻ�ѧ�Լ� |
| C�������Ͷ���������Ư���ԣ������������ϻ����Ư��Ч�� |
| D��Ư�۳���¶���ڿ����л�ʧЧ |
D
��������ˮ�������������ԣ�����̿����ˮ���������������ԣ�A����������������ˮ�������������ᡢ������ȳɷ֣����ƻ�ѧ�Լ�ʱ���ܻ�ʹ�Լ����ʣ�B���������Ͷ��������������������Ӧ��SO2��Cl2��2H2O=H2SO4��2HCl����ʹ��ʧȥƯ���ԣ�C����

��ϰ��ϵ�д�
�����Ŀ