��Ŀ����
��14�֣�ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��3��ʵ���������� ��
�� ��
�� ��
�� ��4����Һ������ʵ����
��4����Һ������ʵ���� ��Һ���������õ���
��
��Һ���������õ���
��
��4��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
|
|

������ɴ�ʵ����ƣ����У�V1= ��V6= ��V9= ��
��ʵ��E�еĽ����� ɫ��
�۸�ͬѧ���ó��Ľ���Ϊ������������ ��Һʱ���������������ʻ�����ߡ����������
��Һʱ���������������ʻ�����ߡ���������� ��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��
��
��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��
��
��1��Zn+CuSO4=ZnSO4+Cu Zn+H2SO4=ZnSO4+H2��
��2��CuSO4��Zn��Ӧ������Cu��Zn�γ�Cu /Zn��أ��ӿ�����������������
��3��Ag2SO4
��4�����߷�Ӧ�¶ȡ��ʵ����������Ũ�ȡ�����п���ıȱ������
��5����30 10 17.5 �ڰ���
�۵�����һ������CuSO4�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ����
����������1��п�ܰ�����ͭ�û���ͭ���Ӷ�����ԭ��أ�����п�Ǹ�����ͭ���������ӿ췴Ӧ���ʣ���������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��Zn+CuSO4=ZnSO4+Cu ��
Zn+H2SO4=ZnSO4+H2����
��2������1��
��3�����Ľ���������п�ģ����Ժ�����ͭ�������Ƶ�����������
��4��������������Է�Ӧ���ʵ�Ӱ�졣���˿���ͨ��ԭ����⣬������ͨ���ı�Ũ�ȡ��¶ȣ�������ȡ������߷�Ӧ�¶ȡ��ʵ����������Ũ�ȡ�����п���ıȱ������
��5���ٽ�һ���о�����ͭ�����������������ʵ�Ӱ�죬�������Ũ��Ӧ������ͬ�ģ�����V1=30ml����������ͭ��Һ������仯�ص��֪�������һ����ǰ���2��������V6=10ml������Fʵ���֪������ͭ��ˮ�����֮����20ml������V9=20ml��2.5ml��17.5ml��
�ڽ�������ɫ�ǰ���ɫ�ġ�
����Ϊ������һ������CuSO4�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ���������Է�Ӧ���ʻή�͡�

ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
(1)����ʵ���з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
__________________________________________��
(2)��������ͭ��Һ����Լӿ������������ʵ�ԭ����_____________________________��
(3)ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ����������ʵ����CuSO4��Һ���������õ���________��
(4)Ҫ�ӿ�����ʵ���������������ʣ����ɲ�ȡ�Ĵ�ʩ��___________________________
____________________________________________________________ (�����ּ���)��
(5)Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ�е�ʵ�飺
|
���� ʵ�� �����Һ������ |
A |
B |
C |
D |
E |
F |
|
4 mol/L H2SO4��mL�� |
30 |
V1 |
V2 |
V3 |
V4 |
V5 |
|
����CuSO4��Һ��mL�� |
0 |
0.5 |
2.5 |
5 |
V6 |
20 |
|
H2O��mL�� |
V7 |
V8 |
V9 |
V10 |
10 |
0 |
�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���������У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
������ɴ�ʵ����ƣ����У�V1��__________��V6��__________��V9��________��
�ڷ�Ӧһ��ʱ���ʵ��A�еĽ�����________ɫ��ʵ��E�еĽ�����________ɫ��
�۸�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��_____________________________________________________________________________________________________________________________��
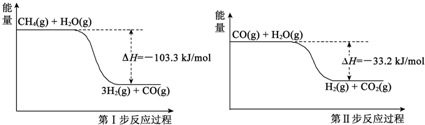 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮 ��
�� ��
�� ��
�� ��4����Һ������ʵ����
��4����Һ������ʵ���� ��Һ���������õ���
��
��Һ���������õ���
��