��Ŀ����
 ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2ʱ���Կ�������ʯ��ˮ�ȱ���Ǻ���������ͨ��SO2û�п�������ʯ��ˮ����ǵ�������˼��������ͬѧ����ͼ��װ�ã��������ռ���ע�����У������ؽ���������ֱ�ͨ�����ʯ��ˮ�У����ܿ�������ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�������������Ա�ͨ��CO2����������죮
ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2ʱ���Կ�������ʯ��ˮ�ȱ���Ǻ���������ͨ��SO2û�п�������ʯ��ˮ����ǵ�������˼��������ͬѧ����ͼ��װ�ã��������ռ���ע�����У������ؽ���������ֱ�ͨ�����ʯ��ˮ�У����ܿ�������ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�������������Ա�ͨ��CO2����������죮
��1������ƿ��װ�����������ƣ�д����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ��______��
��2���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��ʱ��ͨ��SO2������ʯ��ˮ���ܳ��ֻ��ǵ�ԭ�������______��д����ʱ�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��______��
��3����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��SO2��SO2��ʹ����ʯ��ˮ�ȱ���Ǻ����������CO2���ԭ����______��
��4����ͼһ����SO2�����ʯ��ˮ��Ӧ��ʵ��ʱ��Ϊ��ֹSO2��Ⱦ������Ӧ��ʢ�й���Ũ�ռ���Һ����������SO2��д�������ڷ�����Ӧ�Ļ�ѧ����ʽ��______��
�ʴ�Ϊ��H2SO4+Na2SO3=Na2SO4+SO2��+H2O��
��2����������������ʯ��ˮ��Ӧ�������������������ᷢ��CaSO3+H2O+SO2=Ca��HSO3��2�����������������ˮ�����ԣ���ʵ�����ͨ���������Ͽ�϶࣬�����ʯ��ˮ��ԱȽ��٣����߷�Ӧʱֱ��������������ˮ����ʽ�Σ�����������������ʱ��Ӧ�����ӷ���ʽΪ��SO2+OH-=HSO3-��
�ʴ�Ϊ�������ʯ��ˮ�Ƚ��٣�ͨ���������Ͽ�϶࣬���߷�Ӧʱֱ��������������ˮ����ʽ�Σ���������������SO2+OH-=HSO3-��
��3��1���ˮ���ܽ��������Ϊ40�����1���ˮ���ܽ������̼Ϊ2��������ԣ�SO2��ˮ���ܽ�ȱ�CO2������Һ�У��μӷ�Ӧ�Ķ�������ȶ�����̼�ࣻ����������ˮ��Ӧ���������ᣬ������̼��ˮ��Ӧ����̼�ᣬ���������Ա�̼��ǿ����ͬ�¶ȡ�ͬ�������Һ�У�l mol��������������H+����Ũ�ȴ���l mol̼����������H+����Ũ�ȣ���Ӧ��ǰ�ߵ�����������Ӷ࣬��Ӧ�죻��ͬ�����£���ͬ�����ʵ�����Ӧ����Ӧ�죬��˵��SO2���������CO2�죬���ԣ�1mol SO2������ʯ��ˮ��Ӧ����CaSO3�����ʴ���1mol CO2������ʯ��ˮ��Ӧ����CaCO3��������ȷ����ͬ�����£���ͬ�����ʵ�����������������ܽ�ÿ죬˵��SO2���������CO2�죬���ԣ�1molCaSO3��1mol SO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HSO3��2�����ʴ���1molCaCO3��1molCO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HCO3��2�����ʣ�
�ʴ�Ϊ��SO2��ˮ���ܽ�ȱ�CO2������Һ�У��μӷ�Ӧ�Ķ�������ȶ�����̼�ࣻ����������ˮ��Ӧ���������ᣬ������̼��ˮ��Ӧ����̼�ᣬ���������Ա�̼��ǿ����ͬ�¶ȡ�ͬ�������Һ�У�l mol��������������H+����Ũ�ȴ���l mol̼����������H+����Ũ�ȣ���Ӧ��ǰ�ߵ�����������Ӷ࣬��Ӧ�죻1mol SO2������ʯ��ˮ��Ӧ����CaSO3�����ʴ���1mol CO2������ʯ��ˮ��Ӧ����CaCO3�����ʣ�1molCaSO3��1mol SO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HSO3��2�����ʴ���1molCaCO3��1molCO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HCO3��2�����ʣ�
��4��Ϊ��ֹSO2��Ⱦ������Ӧ��ʢ�й���Ũ�ռ���Һ����������SO2����Ӧ�Ļ�ѧ����ʽΪ��SO2+2NaOH=Na2SO3+H2O���ʴ�Ϊ��SO2+2NaOH=Na2SO3+H2O��
��������1������������������Ʒ�Ӧ���������ơ����������ˮ��ԭ���غ���ƽд����
��2��ͨ��SO2������ʯ��ˮ���ܳ��ֻ��ǵ�ԭ������Ƕ�������෴Ӧ��������ˮ����������ƣ�
��3��SO2��ˮ���ܽ�ȱ�CO2�����������Ա�̼��ǿ����ͬ�¶ȡ�ͬ�������Һ�У�l mol��������������H+����Ũ�ȴ���l mol̼����������H+����Ũ�ȣ���ͬ�����£�1mol SO2������ʯ��ˮ��Ӧ����CaSO3�����ʴ���1mol CO2������ʯ��ˮ��Ӧ����CaCO3�����ʣ���ͬ�����£�1molCaSO3��1mol SO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HSO3��2�����ʴ���1molCaCO3��1molCO2���γɵ�H+���ӣ��ܽ��γɿ�����Ca��HCO3��2�����ʣ�
��4����������������������ͼӦ�����գ�
������������Ҫ�����˶�����������ʣ���������ն����������������Ƶķ�Ӧ��ԭ������Ŀ�Ѷ��еȣ�

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д���12�֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣

��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��
ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
_________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��
SO2��SO2�������ǡ�����������CO2
���ԭ����______________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ�
����װ��Ӧ���θĽ���
_____________________________________________��
��4�������������ʵ������ʯ��ˮ�ȱ�����ٳ��塱���������ʯ��ˮ��Ũ���йء�Ϊ��̽��CO2ͨ�����ʯ��ˮ�е�ʵ��������������ݣ�
�� 20��ʱ��Ca(OH)2 ���ܽ��Ϊ��0.165g/100gˮ��
�� ��ͬŨ��ʯ��ˮ����CaCO3�������
| ����ʯ��ˮ��ˮ������� | 1:0 | 1:1 | 1:2 | 1:3 | 1:5 | 1:7 |
| ������CaCO3���������g/100ˮ�� | A | 0.110 | 0.073 | 0.055 | 0.037 | 0.028 |
�� �ϱ���A= g/100gˮ
�� ��1.01��105Pa CO2ѹ���£�CaCO3���ܽ��
| ����ѧ�¶�/K | 282 | 298 | 308 |
| CaCO3�ܽ�ȣ�g/100ˮ�� | 0.130 | 0.094 | 0.076 5 |
�� �ڲ�ͬѹǿ��CO2���£�CaCO3�ܽ�ȣ�18�棩
| P(CO2)/Pa | 0 | 1.40��104 | 9.95��104 |
| CaCO3�ܽ�ȣ�g/100ˮ�� | 0.001 3 | 0.023 3 | 0.108 6 |
��������������ݻش��������⣺
���ɱ���ͱ�����֪CaCO3�ܽ�ȵı仯�����ǣ�
�����������ݿ��Եó����ۣ����۲쵽��ʯ��ˮ�ȱ�����ٳ������������Ҫ��ʵ�������ǣ�
��12�֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣
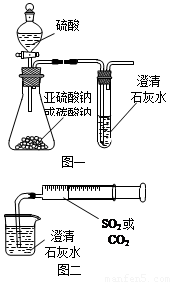
��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��
ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
_________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��
SO2��SO2�������ǡ�����������CO2
���ԭ����______________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ�
����װ��Ӧ���θĽ���
_____________________________________________��
��4�������������ʵ������ʯ��ˮ�ȱ�����ٳ��塱���������ʯ��ˮ��Ũ���йء�Ϊ��̽��CO2ͨ�����ʯ��ˮ�е�ʵ��������������ݣ�
�� 20��ʱ��Ca(OH)2 ���ܽ��Ϊ��0.165g/100gˮ��
�� ��ͬŨ��ʯ��ˮ����CaCO3�������
|
����ʯ��ˮ��ˮ������� |
1:0 |
1:1 |
1:2 |
1:3 |
1:5 |
1:7 |
|
������CaCO3���������g/100ˮ�� |
A |
0.110 |
0.073[��Դ:Zxxk.Com] |
0.055 |
0.037 |
0.028 |
�� �ϱ���A= g/100gˮ
�� ��1.01��105Pa CO2ѹ���£�CaCO3���ܽ��
|
����ѧ�¶�/K |
282 |
298 |
308 |
|
CaCO3�ܽ�ȣ�g/100ˮ��[��Դ:ѧ#��#��Z#X#X#K] |
0.130 |
0.094 |
0.076 5 |
�� �ڲ�ͬѹǿ��CO2���£�CaCO3�ܽ�ȣ�18�棩
|
P(CO2)/Pa |
0 |
1.40��104 |
9.95��104 |
|
CaCO3�ܽ�ȣ�g/100ˮ�� |
0.001 3 |
0.023 3 |
0.108 6 |
��������������ݻش��������⣺
���ɱ���ͱ�����֪CaCO3�ܽ�ȵı仯�����ǣ�
�����������ݿ��Եó����ۣ����۲쵽��ʯ��ˮ�ȱ�����ٳ������������Ҫ��ʵ�������ǣ�

 ���֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2��������������˼�����ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2ʱ�����������Ա�ͨ��CO2�졣
���֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2��������������˼�����ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2ʱ�����������Ա�ͨ��CO2�졣