��Ŀ����
����Ŀ�������(![]() )���Ʊ��й���֬����Ҫԭ�ϣ�ij�����ֲ�Ʒ�к��б����ἰ�۱���ϩ�����������������
)���Ʊ��й���֬����Ҫԭ�ϣ�ij�����ֲ�Ʒ�к��б����ἰ�۱���ϩ�����������������
���� | ��Է������� | �۵�(��) | �е�(��) | ˮ���ܽ��(25��) |
����ȩ | 106 | -26 | 179.62 | �� |
�۱���ϩ | 104n | 83.1~105 | 240.6 | ���� |
����� | 148 | 135 | 300 | ��(��ˮ������) |
ʵ�����ᴿ�����IJ��輰װ������(����װ��δ����)���Իش�������⣺
2g�ֲ�Ʒ��30mL��ˮ�Ļ����![]()
![]() ��Һ
��Һ![]()
![]() ����
����
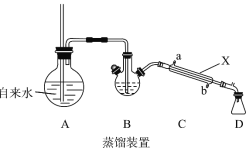
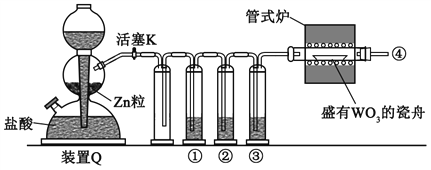
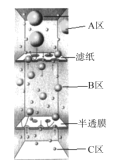
(1)װ��A�г��������ܵ�������_________�������ʹ����ȩ ��ˮ�����뿪ĸҺ������װ����������Ҫ���ȵ�������____________(����ĸ A��B��C��D�ش�)��
(2)����X��������_______����װ������ˮӦ��___________��(��a��b)ͨ�롣
(3)������У�10%NaOH��Һ��������___________���Ա���˳�ȥ�۱���ϩ���ʡ�
(4)������У�֤��ϴ�Ӹɾ�����ѷ�����________������Ʒ�л���������NaCl����һ���ᴿ�������ᾧ�巽��Ϊ_________________��
(5)����ʵ�������ֲ�Ʒ���и�������50%���Ӽ��ܽ�ʱ��ʧ�����10%������ʱ���صõ���Ʒ0.6g�������Ʋ�����ʧ��������ᷴӦ�IJ���ԼΪ_____��(�����ȷ��0.1%)��
���𰸡�ƽ����ѹ AB ������ b �������ת��Ϊ������ˮ��������� �ò�˿պȡ���һ��ϴ��Һ������ɫ��Ӧ�����������ɫ����ϴ�Ӹɾ� �ؽᾧ 66.7
��������
�ֲ�Ʒͨ��ˮ����������ȩ�е�ϵͣ�����ˮ�����ݳ�������NaOH�������ת��Ϊ������ƣ�����ˮ�У����۱���ϩ������ˮ�����˳�ȥ���õ����������Һ������HCl�ữ���������ת��Ϊ����ᣬ����������ܽ�Ƚϵͣ�����Һ���������������ɵõ�����ᡣ
(1)A�ṩˮ���������������ܿ���ƽ��Բ����ƿ�ڲ���ѹǿ�����Ĵ���ѹ���ڼ�����й����е��ܿ���Ԥ��Բ����ƿ�ڲ�ѹǿ���ߵ��·�����ȫ�¹ʡ����������ܵ�������ƽ����ѹ��
A������ṩˮ������B����Ҫ������ά�ֱ���ȩ���е�179.62�棩�ķ��ڣ��ñ���ȩ��ˮ�����ݳ��������Ҫ���ȵ�������AB��
(2)����XΪֱ�������ܣ���ˮӦ���½��룬��b�ڽ���
(3)���������ˮ������NaOH��Ӧ���ɿ�����ˮ��������ƣ��Ӷ����˳�ȥ�۱���ϩ�����NaOH��Һ�������ǽ������ת��Ϊ������ˮ��������ƣ�
(4)���������HCl��Ӧ����������ᣬͬʱ����NaCl���ɣ�����ϴ�ӵ�Ŀ����ϴȥNaCl�������HCl������֤���Ƿ�ϴ�Ӹɾ����Լ�����һ��ϴ��Һ���Ƿ���Na����CI������������ܣ����ʺϼ��H������Ŀ������������δ����ܣ����ʺϼ���CI������˿�������ɫ��Ӧ����Na��������Ϊ�ò�˿պȡ���һ��ϴ��Һ������ɫ��Ӧ�����������ɫ����ϴ�Ӹɾ���
���������ˮ�����ܣ�NaCl���ܽ�������¶ȵı仯������˿��������ܽ�����¶ȱ仯�IJ����Է��룬�����ؽᾧ���ɲ�ȡ�ؽᾧ������ȥNaCl��
(5)��ʵ�������ֲ�Ʒ���и�������50%�������������ʵ���Ϊ![]() �����ܺ�����90%�������������ʵ���Ϊ
�����ܺ�����90%�������������ʵ���Ϊ![]() ��������ᷴӦ�IJ���ԼΪx%����
��������ᷴӦ�IJ���ԼΪx%����![]() �������x=66.7��
�������x=66.7��