��Ŀ����
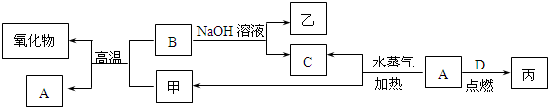
A��B��C��D�ǰ�ԭ��������С�������е�ǰ20��Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ����(���ֲ���δ�г�)

��1��A��___________��C��___________��
��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ��_________________��
��3��E��F��Ӧ�Ļ�ѧ����ʽ��_______________��
��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ��_________________��
��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ��_________________��
��3��E��F��Ӧ�Ļ�ѧ����ʽ��_______________��
��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ��_________________��
(1)̼����
(2)Na2CO3+2HCl=2NaCl+H2O+CO2��
(3)2CO2+2Na2O2=2Na2CO3+O2
(4)Na2O2+S2-+2H2O=4OH-+S��+2Na+
(2)Na2CO3+2HCl=2NaCl+H2O+CO2��
(3)2CO2+2Na2O2=2Na2CO3+O2
(4)Na2O2+S2-+2H2O=4OH-+S��+2Na+

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ