��Ŀ����
2SO2(g)+O2(g) 2SO3 (g)��Ӧ�����������仯��ͼ��ʾ����֪1 molSO2(g) ������Ϊ1 mol SO3ʱ�ġ�H=-99 kJ��mol-1����ش��������⣺
2SO3 (g)��Ӧ�����������仯��ͼ��ʾ����֪1 molSO2(g) ������Ϊ1 mol SO3ʱ�ġ�H=-99 kJ��mol-1����ش��������⣺
 2SO3 (g)��Ӧ�����������仯��ͼ��ʾ����֪1 molSO2(g) ������Ϊ1 mol SO3ʱ�ġ�H=-99 kJ��mol-1����ش��������⣺
2SO3 (g)��Ӧ�����������仯��ͼ��ʾ����֪1 molSO2(g) ������Ϊ1 mol SO3ʱ�ġ�H=-99 kJ��mol-1����ش��������⣺ 
(1)ͼ��A��C�ֱ��ʾ____��____��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿_________��
(2)ͼ�С�H=________ kJ��mol-1��
(3)V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ_________��
(2)ͼ�С�H=________ kJ��mol-1��
(3)V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ_________��
(1)��Ӧ��������������������û��Ӱ�죻
(2)-198
(3)SO2+V2O5=SO3+2VO2;4VO2+O2=2V2O5
(2)-198
(3)SO2+V2O5=SO3+2VO2;4VO2+O2=2V2O5

��ϰ��ϵ�д�
�����Ŀ
 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol�Ħ�H=��99kJ��mol��1����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol�Ħ�H=��99kJ��mol��1����ش��������⣺
 CO(g)+3H2(g) �SH1="+206.1" kJ/mol
CO(g)+3H2(g) �SH1="+206.1" kJ/mol �����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ��10minʱ���ı��������������� ��
�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ��10minʱ���ı��������������� ��
 ��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������
��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ�ڣ���ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������
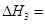 ��800��ʱ����Ӧ�۵Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
��800��ʱ����Ӧ�۵Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
 (��)
(��) (��) b��
(��) b�� 2SO3(g)����H=��96.56 kJ?mol-1���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������ά�־��ȣ����������Խ�����ѧƽ�⡣
2SO3(g)����H=��96.56 kJ?mol-1���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������ά�־��ȣ����������Խ�����ѧƽ�⡣