��Ŀ����
��֪ˮ��25���95��ʱ�������ƽ����������ͼ��ʾ��
��1��25ʱ������9��NaOH��Һ�룽4����Һ��ϣ����������Һ�ģ�7����NaOH��Һ����Һ�������Ϊ
��2��95ʱ����100���1����ijǿ����Һ��1���2��b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���1��ǿ���2֮��Ӧ����Ĺ�ϵ��
��3��95ʱ��pH��2��ijHA��Һ��pH��10��NaOH��Һ�������Ϻ����Һ��pH��5 ����HA�� (�ǿ�ᡱ�����ᡱ)
(1)10:1 (2)a+b=14 (3)����
����:��1��������Һ�ģ�7��˵�����е������Ӻ�OH�������ʵ�����ȣ���V(NaOH)��10��5��V()��10��4������NaOH��Һ����Һ�������Ϊ10�U1��
��2������ͬ��1������ͼ���֪��95��ʱˮ�����ӻ�����Ϊ10��12����Ϻ���Һ�����ԣ���100��10��a��1��10b��12�����a��b��14��
��3��95ʱ��pH��10��NaOH��Һ��Ũ��Ϊ0.01mol/L��pH��2��ijHA��Һ��Ũ��Ӧ�á�0.01 mol/L���������Ϻ�pH��5 ��˵����Һ�����ԣ����������������ֻ�������ᡣ

��ϰ��ϵ�д�
�����Ŀ
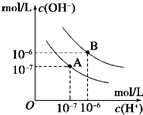 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ������˵������ģ�������
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ������˵������ģ�������| A��A���ߴ���25��ʱˮ�ĵ���ƽ������ | B����95��ʱ��pH=6����Һ������ | C��25��ʱ����10mLpH=12��NaOH��Һ��1mLpH=1��H2SO4��Һ��ϣ�������Һ��pH=7 | D��95��ʱ������������ʵ���Ũ�ȵ�HA��Һ��NaOH��Һ��Ϻ������Һ��pH=6ʱ��˵��HA��Ϊ���� |
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ�� ��1��д��������������ˮ�ĵ��뷽��ʽ�ٱ���������ˮ��
��1��д��������������ˮ�ĵ��뷽��ʽ�ٱ���������ˮ�� ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��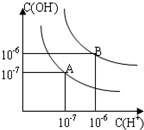 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��