��Ŀ����
9�� ��0.40mol N2O4�������2L�̶��ݻ����ܱ������з������·�Ӧ��N2O4��g��?2NO2��g����H����Tl���T2��ʱ�����NO2�����ʵ�����ʱ��仯��ͼ��ʾ��
��0.40mol N2O4�������2L�̶��ݻ����ܱ������з������·�Ӧ��N2O4��g��?2NO2��g����H����Tl���T2��ʱ�����NO2�����ʵ�����ʱ��仯��ͼ��ʾ����1��Tl��ʱ��40s��80s����N2O4��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.00125mol/��L•s����
��2����H��O�����������������=������
��3���ı��������´ﵽƽ��ʱ��Ҫʹ$\frac{c��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$�ı�ֵ��С���ɲ�ȡ�Ĵ�ʩ��ac������ţ���
a������N2O4����ʼŨ��
b�������¶�
c������������ͨ��NO2
d��ʹ�ø�Ч������
���� ��1��Tl��ʱ��40s��80s�ڶ������������ʵ�����0.40mol��Ϊ0.60mol������v=$\frac{��c}{��t}$������ö���������ʾ��ƽ����Ӧ���ʣ�Ȼ����ݷ�Ӧ�����뻯ѧ�����������ȼ������N2O4��ʾ��ƽ����Ӧ���ʣ�
��2������ͼ�����߱仯��֪��Tl��ʱ��Ӧ���ʴ���T2�棬��T2��ﵽƽ��ʱ�������������ʵ���С��Tl�棬�����¶ȶԻ�ѧƽ���Ӱ���жϸ÷�Ӧ�ķ�Ӧ�ȣ�
��3��a������N2O4����ʼŨ�ȣ�������ѹǿ��ƽ�����������ƶ����ñ�ֵ��С��
b�������¶ȣ�ƽ���������ȵķ�Ӧ�����ƶ���
c������������ͨ��NO2���൱��������ѹǿ��ƽ�����������ƶ����ñ�ֵƫС��
d��ʹ�ø�Ч��������Ӱ�컯ѧƽ�⣮
��� �⣺��1��Tl��ʱ��40s��80s�ڶ������������ʵ�����0.40mol��Ϊ0.60mol�����ö���������ʾ��ʱ��ε�ƽ����Ӧ����Ϊ��v��NO2��=$\frac{\frac{0.60mol-0.40mol}{2L}}{80s-40s}$=0.0025mol/��L•s������ѧ��Ӧ�����뻯ѧ�����������ȣ���v��N2O4��=$\frac{1}{2}$v��NO2��=0.00125mol/��L•s����
�ʴ�Ϊ��0.00125��
��2������ͼ�����߱仯��֪��Tl��ʱ��Ӧ���ʴ���T2�棬���¶ȴ�СΪ��Tl�棾T2�棬����T2��ﵽƽ��ʱ�������������ʵ���С��Tl�棬˵�������¶ȣ�ƽ�����������ƶ�����÷�ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
��3��a������N2O4����ʼŨ�ȣ��൱��������ѹǿ��ƽ�����������ƶ�����$\frac{c��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$�ı�ֵ��С����a��ȷ��
b���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����������ƶ������������Ũ����������������Ũ�ȼ�С���ʸñ�ֵ����b����
c������������ͨ��NO2���൱��������ѹǿ��ƽ�����������ƶ�����������Ũ�ȼ�С������������Ũ�����ñ�ֵ��С����c��ȷ��
d��ʹ�ø�Ч�������Ի�ѧƽ�ⲻӰ�죬��ñ�ֵ���䣬��d����
�ʴ�Ϊ��ac��
���� ���⿼�������ʵ�����ʱ��仯��������ѧƽ���Ӱ�����أ���Ŀ�Ѷ��еȣ���ȷͼ�����߱仯�ĺ���Ϊ���ؼ���ע�����ջ�ѧƽ�⼰��Ӱ�졢��ѧ��Ӧ���ʵļ��㷽����

| A�� | �������е���AgNO3��Һ�������е���Ԫ�أ�Br-+Ag+=AgBr�� | |
| B�� | �ô����ˮ����CaCO3+2H+=Ca2++H2O+CO2�� | |
| C�� | ���ø�ʴ������ӡˢ��·�壺Fe3++Cu=Fe2++Cu2+ | |
| D�� | ʵ��������ͱ��ڴ������������屽�� |
| A�� | ���鲻��ʹ���Ը��������ɫ���ʱ�����Ҳ����ʹ���Ը��������ɫ | |
| B�� | ��ȩ�����ᶼ����̼��˫�����ʶ�����H2�����ӳɷ�Ӧ | |
| C�� | �����ƽṹ�к���ȩ�����ܷ���������Ӧ���ʼ������Ҳ�ܷ���������Ӧ | |
| D�� | �����������ڹ����·�Ӧ������һ�ȼ��飬�ױ��������ڹ����·�Ӧ��Ҫ����2��4-���ȼױ� |
| A�� | KMnO4 | B�� | KClO3 | C�� | MnO2 | D�� | Ca��ClO��2 |
| A�� | NaNO2�������� | B�� | N2�ĵ���ʽ�� | ||
| C�� | ����1 mol N2ʱת��6 mol ���� | D�� | �������ͻ�ԭ��������֮����1��1 |
| A�� | ��ҵ����ˮ����NO2�������3NO2+H2O=2HNO3+NO | |
| B�� | �ð�ˮ��ȥ��ҵԭ���Ȼ���е��Ȼ������ʣ�Fe3++3OH-=Fe��OH��3�� | |
| C�� | ����ʯ�Ҵ���й©��Һ�ȣ�2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O | |
| D�� | ��������ȥˮ�е����������Al3++3H2O?Al��OH��3�����壩+3H+ |
| A�� | 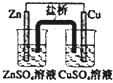 ��װ���У������е�K+����CuSO4��Һ | |
| B�� | 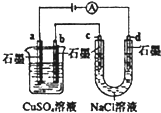 ��ͼװ����b������6.4g����ʱ��d������2.24LH2 | |
| C�� | 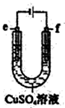 ����ͼװ�þ���ͭʱ��f��Ϊ��ͭ | |
| D�� | 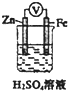 ��ͼװ���е����ص�����Zn����Fe��Fe�����д����������� |
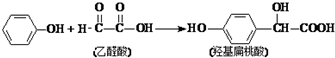
| A�� | �÷�Ӧ��ȡ����Ӧ | |
| B�� | ���Ӻ��ǻ�����������FeCl3��Һ������ɫ | |
| C�� | ��ȩ����H2�ӳɵIJ������ڴ����������γɸ߷��ӻ����� | |
| D�� | 1mol�ǻ�����������3mol NaOH��Ӧ |