��Ŀ����
���Ȼ�����(S2Cl2)�ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵��������ǣ� ��
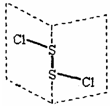
A.S2Cl2�ĽṹʽΪCl��S��S��Cl
B.S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ķǼ��Է���
C.S2Br2��S2Cl2�ṹ���ƣ��۷е㣺S2Br2>S2Cl2
D.S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl
���𰸡�
B
�����������ݽṹͼ��֪���û����ﲻ�ǷǼ��Է��ӣ�����ѡ��A��ȷ��B����ȷ��S2Br2��S2Cl2�γɵľ����Ƿ��Ӿ��壬�۷е�ߵͺͷ��Ӽ���������С�й�ϵ��C��ȷ��S2Cl2��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壬��������SO2��������������������Ȼ���͵���������ѡ��DҲ����ȷ�ģ���ѡB��

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
�����Ŀ