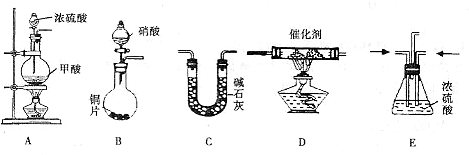
��Ŀ����
����ͼ��ʾװ���ռ�����7������(��ƿλ�ò����ƶ�)��
��H2����Cl2����CH4����HCl����NH3����NO����SO2

(1)����ƿ�Ǹ���ģ�����a�ڽ��������ռ���������________(���ţ���ͬ)����b�ڽ��������ռ���������________��
(2)����ƿ�г���ˮ�����ռ���������________��������________�ڽ��룮
(3)ҽԺ�����������ʱ��������������ƿ�벡�˺������֮�䰲װʢ��ˮ�ĸ�װ�ã����ڹ۲����ݲ�����������Ա���ڹ������ʣ���ʱ����Ӧ��________�ܿڵ��룮

ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺
��һ����ȡ����ͭ
�ٳ�ȡ2 g CuSO4��5H2O��ϸ�����ձ�����10 mL����ˮ�ܽ⣻
��������CuSO4��Һ����μ���NaOH��Һ��ֱ�����ٲ���������Ȼ�����û����ת�Ƶ���������������ȫ����Ϊ��ɫ��
�۽���������û������ˡ�ϴ�ӣ����ɺ���ϸ���á�
�ش��������⣺
��1������ʵ�鲽������Ҫʹ�ò���������__________����ʵ����ţ�������٢�����ĥ��������������������__________��
��2���������ϴ�ӳ����IJ�����________________________________________��
������֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�
����ͼ��ʾװ�ý���ʵ�飬ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�������ݼ��±���

| ʵ����� | KClO3���� | ������������ | �������� |
| �� | 1.2 g | ���������� | |
| �� | 1.2 g | CuO 0.5 g | |
| �� | 1.2 g | MnO2 0.5 g |
�ش��������⣺
��3������ʵ���еġ��������ݡ�ָ__________��
��4����ʵ��װ��ͼ������װ��B�ɸ���ܡ��齺�ܺ�?50 mL?�ζ��ܸ������װ���ɣ��˴����õζ�����__________�����ʽ����ʽ�����ζ��ܣ�
��5����Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סB���齺�ܣ���ȥ������ϵĵ�����Ƥ����____________________��
��6��Ϊ̽��CuO��ʵ������Ƿ�������ã��貹������ʵ�飨����д������������裩��
a.__________________________________________________��
b.֤��CuO�Ļ�ѧ������û�иı䡣
 �������������������������������ռ�����
�������������������������������ռ�����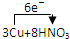 =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O
 ��
��

 CH3COOC2H5 + H2O
CH3COOC2H5 + H2O 

