��Ŀ����
����Ŀ�����仯�����ڹ�ũҵ����������Ҫ�����á�
(1)PԪ���а��ס����ס��������ֳ����ĵ��ʡ�
���ִ���ѧ�У�������_______�ϵ���������������Ԫ�ء�
�ڰ���(P4)������CS2��������ˮ��ԭ����_________��
�ۺ�����һ�ֺ�ɫ�н�������ľ��壬��һ�ֱ�ʯīϩ����������Ͳ��ϡ����ס������Ƿ��Ӿ��壬�����������ʯī�����ƵIJ�״�ṹ������ͼ��ʾ�������йغ������˵������ȷ����_______��
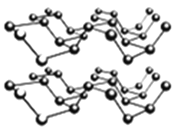
A������������ԭ���ӻ���ʽΪsp3�ӻ�
B���������в����֮����������Ƿ��Ӽ�������
C���������ÿһ������ԭ�Ӷ���ͬһƽ����
D��PԪ�����ֳ����ĵ����У������۷е����
(2)������һ����ĥͿ�ϣ��������������ı��汣���㡣����������廯�����廯���ڸ������������з�Ӧ�ϳɡ�������ľ�������ͼ��ʾ������ʵ����Ϊ��ԭ�ӡ�
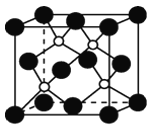
��ش��������⣺
������Ļ�ѧʽΪ_________���þ���ľ���������___________��
�����廯������Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________�����廯����ӵĿռ乹����________���ϳ�����Ļ�ѧ����ʽΪ��___________��
����һ����������ԭ�ӿռ�ѻ���ʽΪ________����ԭ�ӵ���λ��Ϊ________���ýṹ����һ����λ�����ṩ�չ����ԭ����_______����֪������B��Pԭ�ӵ��������Ϊa nm����þ�����ܶȵı���ʽΪ(���軯��)________g/cm3��
���𰸡�ԭ�ӹ��� P4��CS2�ǷǼ��Է��ӣ�H2O�Ǽ��Է��ӣ�������������ԭ����P4������ˮ C BP ԭ�Ӿ��� 1s22s22p63s23p63d104s24p5��[Ar] 3d104s24p5 ƽ�������� PBr3��BBr3��3H2![]() BP��6HBr �����������ܶѻ� 4 B
BP��6HBr �����������ܶѻ� 4 B 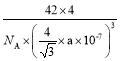
��������
�ִ���ѧ�У�������ԭ�ӹ����ϵ���������������Ԫ�أ����ӵ��ܽ��ԣ�ͨ��������������ԭ�������ľ���ṹ������ʯī����������ԭ������������Ϊ5�����Է���sp3�ӻ������ڳɼ������ܹµ��ӶԵ��ų����ã����Բ�����ԭ�Ӳ���ͬһƽ���ϣ������б��ں��Ͱ��ף����ڲ���ԭ�Ӽ��γɹ��ۼ������Բ���Ӧ�γ�ԭ�Ӿ��塣�����廯���У�Bԭ�ӵ���������ȫ������ɼ������Է��ӳ�ƽ��ṹ���������У���ͼ�п��Կ�����ÿ��Bԭ���γ�4�����ۼ�����B�ļ۵���ֻ��3��������ÿ��Pԭ���ṩ1�Թ¶Ե�����Bԭ��(�ṩ�չ��)�γ���λ����������������ṹ����BP�����У�ÿ��Bԭ����2��Pԭ�ӹ��ɼн�Ϊ109��28���ĵ��������Σ��������Ҷ������ɽ��������߳���B-P������Ķ�����ϵ���Ӷ�����B-P������������ı߳������ս����ܶ���߳��ĵ�����ϵʽ��
(1)���ִ���ѧ�У�����ԭ�ӣ���ʹ��ԭ�ӹ��ף����ù����ϵ���������������Ԫ�ء�
�ڰ���(P4)�ĽṹΪ�������壬Ϊ�Ǽ��Է��ӣ������ڷǼ��Է���CS2�������ڼ��Է���ˮ��ԭ����P4��CS2�ǷǼ��Է��ӣ�H2O�Ǽ��Է��ӣ�������������ԭ����P4������ˮ��
��A������������ԭ������Χ3��Pԭ���γɹ��ۼ���ÿ��Pԭ�ӻ���1�Թ¶Ե��ӣ������ӻ���ʽΪsp3�ӻ���A��ȷ��
B���������в����֮�䲻�γɹ��ۼ�����ͨ�����»�����ϣ����Բ���������Ƿ��Ӽ���������B��ȷ��
C���������ÿһ������ԭ�ӷ���sp3�ӻ������ڹµ��ӶԵ��ų����ã�Pԭ�Ӳ���һ��ƽ���ϣ�C����ȷ��
D��PԪ�����ֳ����ĵ����У����ͺ����γɷ��Ӿ��壬�����ײ�����ԭ�Ӽ��γɹ��ۼ����൱��ԭ�Ӿ��壬���Ժ����۷е���ߣ�D��ȷ��
��ѡC��
��Ϊ��ԭ�ӹ��ף�P4��CS2�ǷǼ��Է��ӣ�H2O�Ǽ��Է��ӣ�������������ԭ����P4������ˮ��C��
(2)�����������У����þ�̯�����������Pԭ�Ӹ���Ϊ![]() =4����Bԭ�Ӹ���Ϊ4������ԭ�Ӹ�����Ϊ1:1����������Ļ�ѧʽΪBP���þ�����ԭ�Ӽ�ȫ���γɹ��ۼ���û�����������������Ծ���������ԭ�Ӿ��塣
=4����Bԭ�Ӹ���Ϊ4������ԭ�Ӹ�����Ϊ1:1����������Ļ�ѧʽΪBP���þ�����ԭ�Ӽ�ȫ���γɹ��ۼ���û�����������������Ծ���������ԭ�Ӿ��塣
�����廯������Ԫ�ػ�̬ԭ�Ӻ�������Ų�Ϊ2��8��18��7�������Ų�ʽΪ1s22s22p63s23p63d104s24p5��[Ar] 3d104s24p5�����廯������У�Bԭ�ӷ���sp2�ӻ��������ڹµ��Ӷԣ����Կռ乹����ƽ�������Σ����廯�����廯���ڸ������������з�Ӧ����BP��HBr���ϳ�����Ļ�ѧ����ʽΪ��PBr3��BBr3��3H2![]() BP��6HBr��
BP��6HBr��
����һ�������У���ԭ�ӵĵ��Ϸ�ʽΪ����һѭ����������ԭ�ӿռ�ѻ���ʽΪ�����������ܶѻ���ÿ����ԭ����Χ�����������ԭ����4����������ԭ�ӵ���λ��Ϊ4���ýṹ����һ����λ�����ṩ�չ����ԭ����B����֪������B��Pԭ�ӵ��������Ϊa nm������ȡһ����BΪ���ĵ��������壬Ȼ�������ȡһ�����������Σ���ʱ�����ļн�Ϊ109��28�����������Ҷ����ɵã�(![]() )2=a2+a2-2aacos109��28�������֪��cos109��28��= -
)2=a2+a2-2aacos109��28�������֪��cos109��28��= -![]() ���Ӷ����x=
���Ӷ����x=![]() nm����þ�����ܶȵı���ʽΪ
nm����þ�����ܶȵı���ʽΪ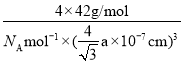 =
=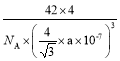 g/cm3��
g/cm3��
��Ϊ��BP��ԭ�Ӿ��壻1s22s22p63s23p63d104s24p5��[Ar] 3d104s24p5��ƽ�������Σ�PBr3��BBr3��3H2![]() BP��6HBr�������������ܶѻ���4��B��
BP��6HBr�������������ܶѻ���4��B��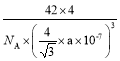 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ж�һЩʵ����ʵ�����۽�����ȷ����
ѡ�� | ʵ����ʵ | ���۽��� |
A | HCl��������ˮ���ܵ��� | HClΪ���ӻ����� |
B | HBr������ǿ��HCl������ | Br�ķǽ����Ա�Clǿ |
C |
|
|
D | HF�ķе����HCl | F�ķǽ����Ա�Clǿ |
A.AB.BC.CD.D
����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)��H2(g) ![]() CO(g)��H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ��
CO(g)��H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ��
t �� | 700 | 800 | 830 | 1 000 | 1 200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��_________________________________��
��2���÷�ӦΪ________��Ӧ(��������������������)��
��3��ij�¶��£������ʵ�ƽ��Ũ�ȷ�����ʽ��3c(CO2)��c(H2)��5c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ______��
��4����830 ��ʱ���������г���1 mol CO��5 mol H2O����Ӧ�ﵽƽ����仯ѧƽ�ⳣ��K______1.0(������������С��������������)��
��5��830 ��ʱ�������еķ�Ӧ�Ѵﵽƽ�⡣�������������������£����������������ƽ��____�ƶ�(����������Ӧ�����������淴Ӧ��������������)��
��6����1 200 ��ʱ����ijʱ��ƽ����ϵ��CO2��H2��CO��H2O��Ũ�ȷֱ�Ϊ2 mol��L��1��2 mol��L��1��4 mol��L��1��4 mol��L��1�����ʱ������Ӧ��ƽ���ƶ�����Ϊ__________(��������Ӧ���������淴Ӧ�������������ƶ���)��