��Ŀ����
��11�֣������������������Լ�����Ӱ���ȣ����Ʊ��������£�
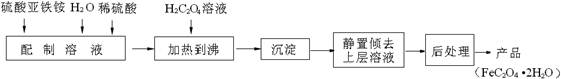
��1������(NH4)2Fe(SO4)2 6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
��2�����ƵõIJ�Ʒ����������н������ط����������ͼ10��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������
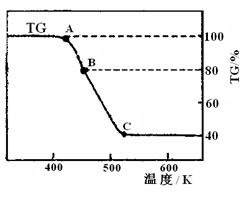
��A B������Ӧ�Ļ�ѧ����ʽΪ
B������Ӧ�Ļ�ѧ����ʽΪ
__________________________________________��
C��ʱ������Ļ�ѧʽΪ______________��
�����о�ѧ����ʵ�������������ɫ�����
H2�����ղ�����Ҳ�����Ĵ����������ɣ�_____________________________
���������һ������ʽ����������ʵ��______________________________ ��
�� ��ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g)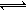 Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��

��1������(NH4)2Fe(SO4)2
 6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________����2�����ƵõIJ�Ʒ����������н������ط����������ͼ10��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������

��A
 B������Ӧ�Ļ�ѧ����ʽΪ
B������Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
C��ʱ������Ļ�ѧʽΪ______________��
�����о�ѧ����ʵ�������������ɫ�����
H2�����ղ�����Ҳ�����Ĵ����������ɣ�_____________________________
���������һ������ʽ����������ʵ��______________________________ ��
�� ��ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g)
 Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________����1������Fe2��ˮ�⣨2�֣�
��2����FeC2O4 2H2O(s)
2H2O(s) FeC2O4(s)+2H2O(g) ��2�֣��� FeO��2�֣�
FeC2O4(s)+2H2O(g) ��2�֣��� FeO��2�֣�
��3FeO+H2O Fe3O4+H2 ��2�֣� ��71.4%��3�֣�
Fe3O4+H2 ��2�֣� ��71.4%��3�֣�
��2����FeC2O4
 2H2O(s)
2H2O(s) FeC2O4(s)+2H2O(g) ��2�֣��� FeO��2�֣�
FeC2O4(s)+2H2O(g) ��2�֣��� FeO��2�֣���3FeO+H2O
 Fe3O4+H2 ��2�֣� ��71.4%��3�֣�
Fe3O4+H2 ��2�֣� ��71.4%��3�֣���

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ
 2NH3(g) ��H����92.4kJ��mol��1����ش�
2NH3(g) ��H����92.4kJ��mol��1����ش� H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��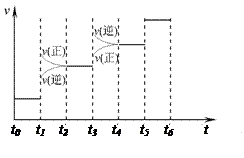
 2CO(g)ƽ�ⳣ��K�ı���ʽΪ ��
2CO(g)ƽ�ⳣ��K�ı���ʽΪ �� 2SO3����ƽ�ⳣ��K=19
2SO3����ƽ�ⳣ��K=19 ��1������Ӧ�ڸ��¶���SO2ת����Ϊ80��ʱ���÷�Ӧ (��ǡ���)�ﵽ��ѧƽ��״̬����δ�ﵽ���� (�����Ӧ�����淴Ӧ��) ������С�
��1������Ӧ�ڸ��¶���SO2ת����Ϊ80��ʱ���÷�Ӧ (��ǡ���)�ﵽ��ѧƽ��״̬����δ�ﵽ���� (�����Ӧ�����淴Ӧ��) ������С� cC(g)+dD(g) ��H = a kJ��mol-1��
cC(g)+dD(g) ��H = a kJ��mol-1�� 2AB(g) �ﵽ��ѧƽ��ı�־�ǣ� ��
2AB(g) �ﵽ��ѧƽ��ı�־�ǣ� �� 2C(g)+xD(g)��5s�ﵽƽ�⡣�ﵽƽ��ʱ��������2molC�����ⶨD��Ũ��Ϊ0.5mol/L�������ж���ȷ����
2C(g)+xD(g)��5s�ﵽƽ�⡣�ﵽƽ��ʱ��������2molC�����ⶨD��Ũ��Ϊ0.5mol/L�������ж���ȷ���� CO2+H2����ƽ�����CO2Ϊ0.75 mol��ͨ��6 molˮ�������ﵽ�µ�ƽ���CO2��H2�����ʵ���֮�Ϳ���Ϊ
CO2+H2����ƽ�����CO2Ϊ0.75 mol��ͨ��6 molˮ�������ﵽ�µ�ƽ���CO2��H2�����ʵ���֮�Ϳ���Ϊ
 2SO3��g�������ﵽƽ�⡣��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת���ʣ�������
2SO3��g�������ﵽƽ�⡣��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת���ʣ������� g��+B��g��
g��+B��g�� nC��g����H=Q���ڲ�ͬ�����·�Ӧ�������C�İٷֺ�
nC��g����H=Q���ڲ�ͬ�����·�Ӧ�������C�İٷֺ� ���ͷ�Ӧ������ʱ��Ĺ�ϵ���ߡ������й�������һ����ȷ���ǣ���
���ͷ�Ӧ������ʱ��Ĺ�ϵ���ߡ������й�������һ����ȷ���ǣ���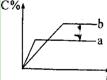
 Ag���̣���Fe3��������Ӧ���ȣ�
Ag���̣���Fe3��������Ӧ���ȣ�