��Ŀ����
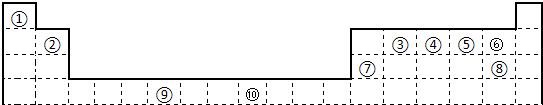
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�
��ش��������⣺
��1����������d����Ԫ����
��2������Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״��������Ϊ
��3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����Ԫ��ԭ�ӵĺ����������ӵŶԵ�����Ϊ
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��

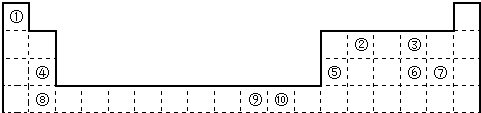
��ش��������⣺
��1����������d����Ԫ����
��
��
�����ţ�����2������Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״��������Ϊ
��
��
���ۺ͢��γɵij���������ľ������������Ӿ���
���Ӿ���
����3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����Ԫ��ԭ�ӵĺ����������ӵŶԵ�����Ϊ
1
1
����Ԫ����Ԫ�آ��γɵķ���X�Ŀռ乹��Ϊ������
������
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
Be��OH��2+2NaOH�TNa2BeO2+2H2O
Be��OH��2+2NaOH�TNa2BeO2+2H2O
����������Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪS����ΪCl����ΪCa����ΪFe����ΪCu��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������
��2��Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ�����ۺ͢��γɵij���������ΪCCl4��
��3��Ԫ�ص����������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2�����Ԫ��ΪN���γɵķ���XΪNH3��
��4��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������
��2��Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ�����ۺ͢��γɵij���������ΪCCl4��
��3��Ԫ�ص����������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2�����Ԫ��ΪN���γɵķ���XΪNH3��
��4��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2��
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪS����ΪCl����ΪCa����ΪFe����ΪCu��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������Cuλ��ds����ֻ��Feλ��d�����ʴ�Ϊ���
��2��Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ�����ۺ͢��γɵij���������ΪCCl4�����ɷ��ӹ��ɣ����ڷ��Ӿ��壬�ʴ�Ϊ���������Ӿ��壻
��3��Ԫ�ص����������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2�����Ԫ��ΪN���������5�����ӣ��¶Ե�����Ϊ1���γɵķ���XΪNH3���ռ乹��Ϊ�����Σ��ʴ�Ϊ��1�������Σ�
��4��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2���÷�ӦΪBe��OH��2+2NaOH�TNa2BeO2+2H2O���ʴ�Ϊ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������Cuλ��ds����ֻ��Feλ��d�����ʴ�Ϊ���
��2��Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ�����ۺ͢��γɵij���������ΪCCl4�����ɷ��ӹ��ɣ����ڷ��Ӿ��壬�ʴ�Ϊ���������Ӿ��壻
��3��Ԫ�ص����������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2�����Ԫ��ΪN���������5�����ӣ��¶Ե�����Ϊ1���γɵķ���XΪNH3���ռ乹��Ϊ�����Σ��ʴ�Ϊ��1�������Σ�
��4��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2���÷�ӦΪBe��OH��2+2NaOH�TNa2BeO2+2H2O���ʴ�Ϊ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
���������⿼��λ�á��ṹ�����ʵ�Ӧ�ã��漰Ԫ�����ڱ���Ԫ�صķ�����Ԫ�����ڱ���Ԫ�ص�λ�á��������͡����ӹ��͡���ѧ��Ӧ�ȣ��ۺ��Խ�ǿ����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ