��Ŀ����
(1)�밴Ҫ����д��ѧ����ʽ��
������ͭ���뷽��ʽΪ
________________________________________________________________________��
������ͭˮ������ӷ���ʽΪ
________________________________________________________________________��
������ͭ��Һ������������Ӧ�����ӷ���ʽΪ____
____________________________________________________________________
________________________________________________________________________��
(2)����NaHSO4��Һ�У���μ���Ba(OH)2��Һ�����ԣ�������Ӧ�����ӷ���ʽΪ____
____________________________________________________________________
________________________________________________________________________��
��������Һ�У������μ�Ba(OH)2��Һ���˲���Ӧ�����ӷ���ʽΪ____
____________________________________________________________________��
����Ba(OH)2��Һ�У���μ���������Һ��Ba2��ǡ����ȫ�������䷴Ӧ�����ӷ���ʽΪ
________________________________________________________________________
________________________________________________________________________��
��������Һ�У������μ�������Һ���˲���Ӧ�����ӷ���ʽΪ___
_____________________________________________________________________��
(3)����������ӷ���ʽ��
��KOH��Һ������Ca(HCO3)2��Һ��Ӧ
________________________________________________________________________��
��KOH��Һ������Ca(HCO3)2��Һ��Ӧ
________________________________________________________________________��
��Ca(OH)2��Һ������NaHCO3��Һ��Ӧ
________________________________________________________________________��
������Ca(OH)2��Һ��NaHCO3��Һ��Ӧ
________________________________________________________________________��
������(1)Ӧ��ע���������෴Ӧ����ʽ��д���ϵIJ�ͬ��(2)Ba(OH)2��NaHSO4�ķ�Ӧ�����⣬Ӧ���ע���ж��ڷ�Ӧ���������ʹ�����Ba(OH)2�������ķ�Ӧ����Ba2����SO![]() ��OH����Al3����������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�(3)����Ca(HCO3)2�������ģ�KOH������Խ��٣�OH����HCO
��OH����Al3����������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�(3)����Ca(HCO3)2�������ģ�KOH������Խ��٣�OH����HCO![]() ��Ӧ����������H2O��CO
��Ӧ����������H2O��CO![]() ����ʣ��HCO
����ʣ��HCO![]() δ��Ӧ��
δ��Ӧ��
�𰸡�(1)��CuSO4===Cu2����SO![]()
��Cu2����H2OCu(OH)2��2H��
��Ba2����SO![]() ��2OH����Cu2��===BaSO4����Cu(OH)2��
��2OH����Cu2��===BaSO4����Cu(OH)2��
(2)��2H����SO![]() ��2OH����Ba2��===BaSO4����2H2O
��2OH����Ba2��===BaSO4����2H2O
Ba2����SO![]() ===BaSO4��
===BaSO4��
��Al3����2SO![]() ��4OH����2Ba2��===2BaSO4����AlO
��4OH����2Ba2��===2BaSO4����AlO![]() ��2H2O��Al3����3AlO
��2H2O��Al3����3AlO![]() ��6H2O===4Al(OH)3��
��6H2O===4Al(OH)3��
(3)��OH����Ca2����HCO![]() ===CaCO3����H2O
===CaCO3����H2O
��2OH����Ca2����2HCO![]() ===CaCO3����2H2O��CO
===CaCO3����2H2O��CO![]()
��Ca2����2OH����2HCO![]() ===CaCO3����2H2O��CO
===CaCO3����2H2O��CO![]()
��HCO![]() ��Ca2����OH��===CaCO3����H2O
��Ca2����OH��===CaCO3����H2O

 ��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
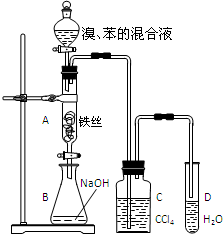 ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�

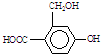 Ϊʵ���������ʵ�ת��
Ϊʵ���������ʵ�ת��