��Ŀ����
����Ŀ�����и������ʴ������Ұ�ͬ���л��ͬλ�ء�ͬϵ�ͬ���칹�塢ͬ�������塢ͬ�����ʵ�˳�����е���( )
��C60��C70�����ʯ��ʯī �����״����Լ����ӡ��ڼ����ӡ��������
��![]() ��
��![]() ��
��![]() ��HOCH2CHO��HOCH2CH2CHO��HOCH2CH2CH2CHO
��HOCH2CHO��HOCH2CH2CHO��HOCH2CH2CH2CHO
�������顢2,2�������� ���״����Ҷ�����������
A.�٢ޢڢۢݢ�B.�ޢۢݢܢ٢�
C.�ܢڢޢ٢ݢ�D.�ޢۢܢڢ٢�
���𰸡�D
��������
��C60��C70�����ʯ��ʯī��̼Ԫ�صIJ�ͬ���ʣ���Ϊͬ�������壻
�ڱ��״����Լ����ӡ��ڼ����ӡ�������ӷ���ʽ��ͬ�ṹ��ͬ����Ϊͬ���칹�壻��![]() ��
��![]() ��
��![]() ����������ͬ����������ͬ����Ϊͬλ�أ�
����������ͬ����������ͬ����Ϊͬλ�أ�
��HOCH2CHO��HOCH2CH2CHO��HOCH2CH2CH2CHO�ṹ���ƣ��ڷ�����������һ�������ɸ�CH2ԭ���ŵĻ������Ϊͬϵ�
�������顢2��2�����������ʽ��ͬ���ṹ��ͬ����ͬ�����ʣ�
�״����Ҷ������������������ǻ�����ͬ���л��
��ѡ��Dѡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������

ѡ�� | X | Y | Z | R |
A | Al | AlCl3 | Al(OH)3 | NaAlO2 |
B | Na | Na2O | Na2O2 | NaOH |
C | H2S | S | SO2 | SO3 |
D | N2 | NH3 | NO | NO2 |
A.AB.BC.CD.D
����Ŀ����1����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______________������֪��H2O��g��=H2O��l������H2����44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������_____________kJ��
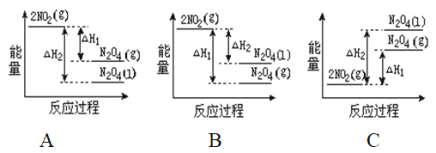
��2����֪��2NO2��g��![]() N2O4��g����H1 2NO2��g��
N2O4��g����H1 2NO2��g��![]() N2O4��l����H2
N2O4��l����H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_____________��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C��s��ʯī����O2��g����CO2��g�� ��H1����393.5 kJ��mol��1
2H2��g����O2��g����2H2O��l�� ��H2����571.6 kJ��mol��1
2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H3����2 599 kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���ʱ䣨�г��ļ���ʽ����___________________________��
��4���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״����壨�ṹ��ʽΪCH3OH���� ��֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��֪CO�е�C��O֮��Ϊ�������ӣ���ҵ�Ʊ��״����Ȼ�ѧ����ʽΪ_________��
����Ŀ������ʵ��������ͻ������ȷ����( )
ѡ�� | ʵ�� | ���ͻ���� |
A | ��ij��Һ����ͨ��CO2���壬�ȳ��ְ�ɫ��״����������ͨ��CO2���壬��ɫ��״�������ܽ⣬֤������Һ�д���AlO2�� | Al(OH)3�������������������̼����Һ |
B | ��������ˮ�м���̼��Ʒ�ĩ����ֽ��裬��ˮ��Ư������ǿ | ��ˮ��HClO�����ʵ���Ũ������ |
C | �������ܽ��ܽ���CCl4�еĵ������� | ��Ϊ�����������ȷ������ |
D | ��Fe(NO3)2��Һ�е��������ữ��H2O2��Һ������Һ��Ϊ��ɫ | �����ԣ�H2O2 > Fe3+ |
A.AB.BC.CD.D
����Ŀ��Ϊ�˲ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȣ�ȷ��ȡM g������Ʒ�����250 mL��Һ���������������ʵ�鷽����
����I��ȡ50.00 mL������Һ�����������������ữ��BaCl2��Һ������I��ϴ�ӡ���������������õ�����������Ϊm1 g
������ȡ50.00 mL������Һ����a mol/L ������KMnO4��Һ���еζ���
ʵ��������¼���������±���
�ζ����� ʵ������ | 1 | 2 | 3 | 4 |
������Һ���/mL | 50.00 | 50.00 | 50.00 | 50.00 |
�ζ��ܳ�����/mL | 0.00 | 0.20 | 0.10 | 0.15 |
�ζ���ĩ����/mL | 20.95 | 21.20 | 20.15 | 21.20 |
(1)����250 mL Na2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܡ�ҩ��_______��________��
(2)����IΪ______________��������Ϊ______________��
(3)�ڷ������еζ��յ���жϷ�����_______________________________��
(4)�ڷ������з��������ӷ�Ӧ����ʽΪ____________________________��
(5)���ݷ��������ṩ�����ݣ�����Na2SO3�Ĵ���Ϊ___________����д�ɷ�����ʽ��
(6)��������������ԭ�ζ������У����´���ҺNa2SO3Ũ�ȱ�С����_____������ţ���
a���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ����ʼ���ӣ��ζ�����ʱ����
b���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ��һ��ʼ�����ݣ��ζ�������û����
c����ʽ�ζ���������ˮ��ϴ��û��������KMnO4��Һ�����ϴ
d����ƿ������ˮ��ϴ��ֱ��װ50.00mL�Ĵ���Һ
e���ζ�����ʱ����ʼʱƽ�ӣ��ζ�����ʱ����
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ��밴Ҫ����д���пհף�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
2 | �� | �� | �� | �� | |||
3 | �� | �� | �� | �� |
(1)��Ԫ�آ٢ڢݢޢߢ��Ӧ������������ˮ�����У�������ǿ�Ļ����������ʽ�ǣ�_________��
(2)д��Ԫ�آڵ�����⻯��Ļ�ѧʽ____________��
(3)�ܢݢޢ�����Ԫ�صļ����Ӱ뾶�Ӵ�С����____________(�����ӷ��ű�ʾ)��
(4)д��Ԫ�آ������������Ԫ�آݵ�����������ˮ���ﷴӦ�����ӷ���ʽ______________________________________��
(5)д��Ԫ�آ۵ij����⻯�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ_______________��


