��Ŀ����
��.����ʵ��������ʵ����ʵ��������ȷ����__________������ţ���
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
��һС������Ƽ���ˮ�к�Ѹ���۳�С��ͣ����ˮ���ζ���������˻˻����������
������100 mL 1.00 mol��L-1 ��NaCl��Һʱ������������ƽ��ȡ5.85 g NaCl���壻
������ܺ���![]() ,
,![]() ����Һ�м�����������ᣬ�ټ���Ba��NO3��2��Һ���ɼ���
����Һ�м�����������ᣬ�ټ���Ba��NO3��2��Һ���ɼ���![]() �Ĵ��ڣ�
�Ĵ��ڣ�
������NaCl��Һ�����õ��϶�NaCl����ʱ����ֹͣ���ȣ������������ɣ�
����AlCl3��Һ�еμ�NaOH��Һ����NaOH��Һ�еμ�AlCl3��Һ��������ͬ��
��.��1����ʵ������6 mol��L-1 ������250 mL������ʱ��������Ͳ���ձ����������⣬�����õ������У�__________��__________��
��2��ʵ������������ı���NaCl��Һ��ͨ�������CO2���Ʊ�NaHCO3���壬Ϊ�˷����NaHCO3���壬�����õ���ͼ��__________������ţ�װ�á�

��3��д������NaHCO3�Ļ�ѧ����ʽ��______________________________��
��.�ڢ�
��.��1��250 mL����ƿ����ͷ�ιܡ���2��C
��3��NaCl��NH3��CO2��H2O====NaHCO3����NH4Cl
����:
��.��Ҫע�������SO2������ˮ��![]() �����Ի����лὫ
�����Ի����лὫ![]() ������
������![]() ��
��

 �ŵ������ϵ�д�
�ŵ������ϵ�д�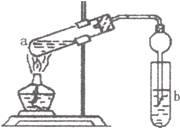 ��1������ʵ��������ʵ����ʵ��������ȷ����
��1������ʵ��������ʵ����ʵ��������ȷ����