��Ŀ����
����Ҫ����գ�
��1����ϵͳ�����������л��CH3-CH2-C��CH3��2-CH��CH3��-C2H5�� ��
��2����д������Ľṹ��ʽ��3��5-����-3-��ϩ�� ��
��3����д�ױ���һ�������º�Ũ���ᡢŨ����ķ�Ӧ����ʽ ����Ӧ������ ��
��4����д����������������ˮ��Һ���ȵķ�Ӧ����ʽ ��Ӧ������ ��
��5����д1-�ȱ��������������Ҵ���Һ���ȵķ�Ӧ����ʽ ��Ӧ������ ��
��1����ϵͳ�����������л��CH3-CH2-C��CH3��2-CH��CH3��-C2H5��
��2����д������Ľṹ��ʽ��3��5-����-3-��ϩ��
��3����д�ױ���һ�������º�Ũ���ᡢŨ����ķ�Ӧ����ʽ
��4����д����������������ˮ��Һ���ȵķ�Ӧ����ʽ
��5����д1-�ȱ��������������Ҵ���Һ���ȵķ�Ӧ����ʽ
��������1����2����������ԭ��
�ٳ�-----ѡ�̼��Ϊ������
�ڶ�-----���ȳ�̼��ʱ��֧�����Ϊ������
�۽�-----��֧�����һ�˱�ţ�
��С-----֧�����֮����С��������ṹ��ʽ�����Ҷ˻���˿��������ϡ���-----��֧�����һ�˱�š���ԭ��
�ݼ�-----��ȡ���������������˵Ⱦ���ʱ���Ӽ�ȡ������ʼ��ţ���ȡ������ͬ���ͰѼ�д��ǰ�棬���ӵ�д�ں��棻
��3�������ϵ���ԭ�ӱ�����ȡ�������������ױ�������ȡ����Ӧ��
��4������±����ˮ������������������NaOHˮ��Һ����ȡ����Ӧ��
��5������±������ȥ�����������������NaOH����Һ������ȥ��Ӧ��
�ٳ�-----ѡ�̼��Ϊ������
�ڶ�-----���ȳ�̼��ʱ��֧�����Ϊ������
�۽�-----��֧�����һ�˱�ţ�
��С-----֧�����֮����С��������ṹ��ʽ�����Ҷ˻���˿��������ϡ���-----��֧�����һ�˱�š���ԭ��
�ݼ�-----��ȡ���������������˵Ⱦ���ʱ���Ӽ�ȡ������ʼ��ţ���ȡ������ͬ���ͰѼ�д��ǰ�棬���ӵ�д�ں��棻
��3�������ϵ���ԭ�ӱ�����ȡ�������������ױ�������ȡ����Ӧ��
��4������±����ˮ������������������NaOHˮ��Һ����ȡ����Ӧ��
��5������±������ȥ�����������������NaOH����Һ������ȥ��Ӧ��
����⣺��1��CH3-CH2-C��CH3��2-CH��CH3��-C2H5������Ϊ��3��3��4-�������飬�ʴ�Ϊ��3��3��4-�������飻
��2��3��5-����-3-��ϩ�Ľṹ��ʽ��CH3CH2C=��CH3��CH2CH��CH3��CH2CH3���ʴ�Ϊ��CH3CH2C=��CH3��CH2CH��CH3��CH2CH3��
��3�������ϵ���ԭ�ӱ�����ȡ�������������ױ�������ȡ����Ӧ������ʽΪ��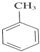 +3HNO3
+3HNO3
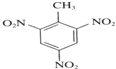 +3H2O��
+3H2O��
�ʴ�Ϊ�� +3HNO3
+3HNO3
 +3H2O��ȡ����Ӧ��
+3H2O��ȡ����Ӧ��
��4��±�����ڼ���ˮ��Һ���������·���ȡ����Ӧ�������������Ҵ��������Ҵ����廯�Ʒ���ʽΪCH3CH2Br+NaOH
CH3CH2OH+NaBr���ʴ�Ϊ��CH3CH2Br+NaOH
CH3CH2OH+NaBr��ȡ����Ӧ��
��5��±�������������ƴ���Һ���������·�����ȥ��Ӧ��1-�ȱ��������������Ҵ���Һ���ȷ�Ӧ����ʽ��CH2ClCH2CH3+NaOH
CH2=CHCH3��+H2O+NaBr���ʴ�Ϊ��CH2ClCH2CH3+NaOH
CH2=CHCH3��+H2O+NaBr����ȥ��Ӧ��
��2��3��5-����-3-��ϩ�Ľṹ��ʽ��CH3CH2C=��CH3��CH2CH��CH3��CH2CH3���ʴ�Ϊ��CH3CH2C=��CH3��CH2CH��CH3��CH2CH3��
��3�������ϵ���ԭ�ӱ�����ȡ�������������ױ�������ȡ����Ӧ������ʽΪ��
 +3HNO3
+3HNO3
| ||
| ���� |
 +3H2O��
+3H2O���ʴ�Ϊ��
 +3HNO3
+3HNO3
| ||
| ���� |
 +3H2O��ȡ����Ӧ��
+3H2O��ȡ����Ӧ����4��±�����ڼ���ˮ��Һ���������·���ȡ����Ӧ�������������Ҵ��������Ҵ����廯�Ʒ���ʽΪCH3CH2Br+NaOH
| ���� |
| ���� |
��5��±�������������ƴ���Һ���������·�����ȥ��Ӧ��1-�ȱ��������������Ҵ���Һ���ȷ�Ӧ����ʽ��CH2ClCH2CH3+NaOH
| �� |
| ���� |
| �� |
| ���� |
���������⿼��������������������Ӧ�á�ȡ������ȥ��Ӧ�ȣ���Ŀ�Ѷ��еȣ�ע������ѡ��λ�ñ�ŵ���ȷѡ���±����ȡ������ȥ��������ͬ��

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ


