��Ŀ����
���������ҹ���������᳧��ȡ�������Ҫԭ�ϣ�ij��ѧ��ȤС���ij������ʯ����Ҫ�ɷ�ΪFeS2����������ʵ��̽����
[ʵ��һ]�ⶨ����������Ԫ�صĺ���
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��

��Ӧ��������ƿ�е���Һ�������´�����

�������ۣ�
��1�����У�ʯӢ���з����ķ�Ӧ����ʽΪ______��
��2�����У�Ϊ�ж�����BaCl2�Ƿ��������ͬѧ���������ʵ�鷽�������к�������______
A��ȡ�����ϲ���Һ�������μ�BaCl2��������������˵��BaCl2�ѹ�����
B��ȡ�����ϲ���Һ�������μ�ϡH2SO4��������������˵��BaCl2�ѹ�����
C������Һ��������μ�BaCl2��������������˵��BaCl2�ѹ�����
D������Һ��������μ�ϡH2SO4��������������˵��BaCl2�ѹ�����
��3���û���������Ԫ�ص���������Ϊ______��
[ʵ���]�ⶨ����������Ԫ�صĺ���
��������ϡ�����ܽ�ʯӢ���еĹ��������
�ڼӻ�ԭ��ʹ��Һ�е�Fe3+��ȫת��ΪFe2+���ˡ�ϴ�ӣ�
�۽���Һϡ����250mL��
��ȡϡ��Һ25.00mL����Ũ��Ϊ0.10mol/L������KMnO4��Һ�ζ���ʵ��ƽ������KMnO4��Һ���ΪV mL��
�������ۣ�
��4�����У���Ҫ�õ�����Ҫ������______��
��5�����У���ѡ����������ԭ��������Ϊ������______��������______��
��6������������Ԫ�ص���������Ϊ______��
�⣺ʵ��һ����1�����У�ʯӢ���ж���������������Ӧ�������������������Ӧ����ʽΪ4FeS2+11O2 2Fe2O3+8SO2��
2Fe2O3+8SO2��
�ʴ�Ϊ��4FeS2+11O2 2Fe2O3+8SO2��
2Fe2O3+8SO2��
��2������BaCl2��Һ�ǽ��������ת��Ϊ���ᱵ�������ж�����BaCl2�Ƿ���������ǣ�����Һ�����ȡ�����ϲ���Һ�������μ�BaCl2��������������˵��BaCl2�ѹ�����
��ѡC��
��3��m2g�����ᱵ�����������ᱵ�����ʵ���Ϊ =
= mol��������Ԫ���غ��֪����������Ԫ�ص�����Ϊ
mol��������Ԫ���غ��֪����������Ԫ�ص�����Ϊ mol��32g/mol=
mol��32g/mol= g����������������Ԫ�ص���������Ϊ
g����������������Ԫ�ص���������Ϊ ��100%=
��100%= ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ�� ��100%��
��100%��
ʵ�������4��������ǵζ�����Ҫ�õ�����Ҫ�����У�����̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
�ʴ�Ϊ������̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
��5���÷���ͨ���ζ��������ɵ�Fe2+��ȷ������������Ԫ�ص�������������������ԭ����������Ԫ�ص��������²ⶨ������������Ԫ�ص���������ƫ�ߣ��ʲ�����������ԭ����
�ʴ�Ϊ������������������ԭ����������Ԫ�ص�����
��6����m1g����������Ԫ�ص�����Ϊa������������Ԫ�ص��������������ӵ���������
5Fe2+��MnO4-
280g 1mol
a 0.001VL��0.1mol/L��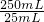 =0.001Vmol
=0.001Vmol
����a= ��280g/mol=0.28Vg
��280g/mol=0.28Vg
��������������Ԫ�ص���������Ϊ ��100%=
��100%= %���ʴ�Ϊ��
%���ʴ�Ϊ�� %��
%��
������ʵ��һ����1�����У�ʯӢ���ж���������������Ӧ�������������������
��2������BaCl2��Һ�ǽ��������ת��Ϊ���ᱵ����������Һ�����ȡ�����ϲ���Һ�������μ�BaCl2�������Ƿ�����������ӣ�������������˵��BaCl2�ѹ�����
��3��m2g�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ�������������Ԫ�ص�����������
ʵ�������4��������ǵζ�����Ҫ�õ�����Ҫ�����У�����̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
��5���÷���ͨ���ζ��������ɵ�Fe2+��ȷ������������Ԫ�ص�������������������ԭ����������Ԫ�ص�����
��6������������Ԫ�ص��������������ӵ����������ݵ���ת���غ�ɵù�ϵʽ5Fe2+��MnO4-���ݴ˼�������������Ԫ�ص�����������������Ԫ�ص�����������
���������⿼��ѧ����ʵ��ԭ�������⡢ʵ������������Բ��������ۡ�������ԭ�ζ�����ѧ����ȣ��ۺ��Խϴ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������
 2Fe2O3+8SO2��
2Fe2O3+8SO2���ʴ�Ϊ��4FeS2+11O2
 2Fe2O3+8SO2��
2Fe2O3+8SO2����2������BaCl2��Һ�ǽ��������ת��Ϊ���ᱵ�������ж�����BaCl2�Ƿ���������ǣ�����Һ�����ȡ�����ϲ���Һ�������μ�BaCl2��������������˵��BaCl2�ѹ�����
��ѡC��
��3��m2g�����ᱵ�����������ᱵ�����ʵ���Ϊ
 =
= mol��������Ԫ���غ��֪����������Ԫ�ص�����Ϊ
mol��������Ԫ���غ��֪����������Ԫ�ص�����Ϊ mol��32g/mol=
mol��32g/mol= g����������������Ԫ�ص���������Ϊ
g����������������Ԫ�ص���������Ϊ ��100%=
��100%= ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ�� ��100%��
��100%��ʵ�������4��������ǵζ�����Ҫ�õ�����Ҫ�����У�����̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
�ʴ�Ϊ������̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
��5���÷���ͨ���ζ��������ɵ�Fe2+��ȷ������������Ԫ�ص�������������������ԭ����������Ԫ�ص��������²ⶨ������������Ԫ�ص���������ƫ�ߣ��ʲ�����������ԭ����
�ʴ�Ϊ������������������ԭ����������Ԫ�ص�����
��6����m1g����������Ԫ�ص�����Ϊa������������Ԫ�ص��������������ӵ���������
5Fe2+��MnO4-
280g 1mol
a 0.001VL��0.1mol/L��
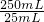 =0.001Vmol
=0.001Vmol����a=
 ��280g/mol=0.28Vg
��280g/mol=0.28Vg��������������Ԫ�ص���������Ϊ
 ��100%=
��100%= %���ʴ�Ϊ��
%���ʴ�Ϊ�� %��
%��������ʵ��һ����1�����У�ʯӢ���ж���������������Ӧ�������������������
��2������BaCl2��Һ�ǽ��������ת��Ϊ���ᱵ����������Һ�����ȡ�����ϲ���Һ�������μ�BaCl2�������Ƿ�����������ӣ�������������˵��BaCl2�ѹ�����
��3��m2g�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ�������������Ԫ�ص�����������
ʵ�������4��������ǵζ�����Ҫ�õ�����Ҫ�����У�����̨�����Եζ��ܡ��ձ����ζ��ܼС���ƿ��
��5���÷���ͨ���ζ��������ɵ�Fe2+��ȷ������������Ԫ�ص�������������������ԭ����������Ԫ�ص�����
��6������������Ԫ�ص��������������ӵ����������ݵ���ת���غ�ɵù�ϵʽ5Fe2+��MnO4-���ݴ˼�������������Ԫ�ص�����������������Ԫ�ص�����������
���������⿼��ѧ����ʵ��ԭ�������⡢ʵ������������Բ��������ۡ�������ԭ�ζ�����ѧ����ȣ��ۺ��Խϴ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ






 2Fe2O3
+ 8SO2
2Fe2O3
+ 8SO2



