��Ŀ����
����Ŀ������������![]() �����������Ͻ���Բ��ϡ������ȡ�Ϊ���������Դ����ʵ������̽�����ú��ܷ�������
�����������Ͻ���Բ��ϡ������ȡ�Ϊ���������Դ����ʵ������̽�����ú��ܷ�������![]() �ȣ����Ʊ����������ܣ������������£�
�ȣ����Ʊ����������ܣ������������£�

�ش��������⣺
��1��![]() �ĵ���ʽΪ_______________________��
�ĵ���ʽΪ_______________________��
��2��������ˮϴ����Ŀ��Ϊ______________________________________________��
��3���������ʱ��![]() ������Ӧ�����ӷ���ʽΪ_____________________________________________������1����Ҫ�ɷֵĻ�ѧʽΪ_______________________��
������Ӧ�����ӷ���ʽΪ_____________________________________________������1����Ҫ�ɷֵĻ�ѧʽΪ_______________________��
��4��������ˮϴҺ���к���![]() �IJ���������Ϊ______________________________________________�����������ж��Ӧ�ù��˲����ò���������Ҫ����������_____________________��
�IJ���������Ϊ______________________________________________�����������ж��Ӧ�ù��˲����ò���������Ҫ����������_____________________��
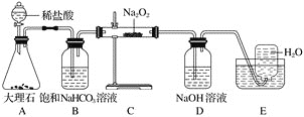
��5������װ���У��ʺϽ�������������������_____________________����ѡ����ĸ����
A. B.
B. C.
C. D.
D.
��6������![]() ���������������ø���������
���������������ø���������![]() ����������Ļ�ѧʽΪ________________�������������������ɵ������ܲ������ѭ������ù���_______________�������������������������μӷ�Ӧ��
����������Ļ�ѧʽΪ________________�������������������ɵ������ܲ������ѭ������ù���_______________�������������������������μӷ�Ӧ��
���𰸡�![]() ��ȥ���ܷ������������ Co2O3+4H++
��ȥ���ܷ������������ Co2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O CaSO4 �ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ �ձ���©���������� C Co2O7 ��
+2H2O CaSO4 �ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ �ձ���©���������� C Co2O7 ��
��������
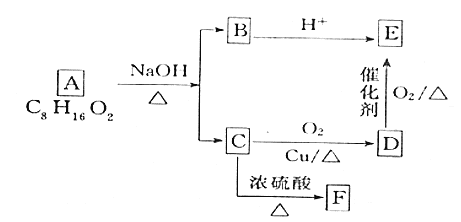
��������ʾ��ͼ������֪�����ܷ������ȵĴ�����Һϴȥ��������ۣ���ˮK2O�ܽ⣬ͬˮϴҺ������ϴ�Ӻ����������Һ��Na2SO3������������ʱ������ӦCo2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��ͬʱCaO��H2SO4��Ӧ����CaSO4���õ�����CaSO4������1����Ҫ��CoSO4����Һ1���ټ���NH4HCO3������ӦCoSO4+NH4HCO3=CoCO3+NH4HSO4�õ�CoCO3�ij��������������պ�õ�����������(CoxOy)���ݴ˷������
+2H2O��ͬʱCaO��H2SO4��Ӧ����CaSO4���õ�����CaSO4������1����Ҫ��CoSO4����Һ1���ټ���NH4HCO3������ӦCoSO4+NH4HCO3=CoCO3+NH4HSO4�õ�CoCO3�ij��������������պ�õ�����������(CoxOy)���ݴ˷������
(1)K2O�����ӻ����K+��O2-�γ����Ӽ��������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)�ȵĴ�����Һ���н�ǿ�ļ��ԣ���ʹ��������ˮ��Ϊ������ˮ�����ʣ�����ˮϴ��ȥ���ʴ�Ϊ����ȥ���ܷ�����������ۣ�
(3)�������ʱ��Co2O3�����������±���ԭ����Ӧ�����ӷ���ʽΪCo2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��ͬʱ��CaO��H2SO4��Ӧ�����ܽ��С��CaSO4���ʴ�Ϊ��Co2O3+4H++
+2H2O��ͬʱ��CaO��H2SO4��Ӧ�����ܽ��С��CaSO4���ʴ�Ϊ��Co2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��CaSO4��
+2H2O��CaSO4��
(4)����K+������ɫ��Ӧ������������Ϊ�ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ���������õ���Ҫ�����������ձ���©���Ͳ��������ʴ�Ϊ���ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ���ձ���©������������
(5)�����ա�����Ӧ�������н��У�Cѡ����ȷ���ʴ�Ϊ��C��
(6)5.95gCoCO3�����ʵ���Ϊ![]() �����������к���Ԫ�ص�����Ϊ0.05mol��59g��mol-1=2.95g������Ԫ�ص�����Ϊ4.07g-2.95g=1.12g����0.07mol����˸�����������Co��O�ĸ�����Ϊ5��7���������ܵĻ�ѧʽΪCo2O7��CoCO3ת��ΪCo5O7ʱCoԪ�صĻ��ϼ����ߣ����������μӷ�Ӧ���ʴ�Ϊ��Co2O7���С�
�����������к���Ԫ�ص�����Ϊ0.05mol��59g��mol-1=2.95g������Ԫ�ص�����Ϊ4.07g-2.95g=1.12g����0.07mol����˸�����������Co��O�ĸ�����Ϊ5��7���������ܵĻ�ѧʽΪCo2O7��CoCO3ת��ΪCo5O7ʱCoԪ�صĻ��ϼ����ߣ����������μӷ�Ӧ���ʴ�Ϊ��Co2O7���С�

 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�����Ŀ�����¶�Ϊ20 �棬Ũ��Ϊ1.0 mol/L��H2SO4��Һ��2.2 mol/L�ļ���Һ��50 mL���[��Һ�ܶȾ�Ϊ1g/mL��������Ϊ4.18 kJ/(kg����)]�����������������Һ���¶ȱ仯�������£�
��Ӧ�� | ��ʼ�¶�t1 �� | ��ֹ�¶�t2 �� |
H2SO4��NaOH | 20 | 33.6 |
H2SO4��NH3��H2O | 20 | 32.6 |
��1����ӦNH3��H2O(aq)![]() NH4+ (aq)��OH��(aq)���ʱ�Լ____��
NH4+ (aq)��OH��(aq)���ʱ�Լ____��
��2��������������ʵ�������к��ȣ���H1��___kJ/mol����H2��__kJ/mol��
��3���ɱ�����ۿ�Ԥ�⽫��1���е�1 mol/L��H2SO4��Һ����2mol/L��CH3COOH��Һ����ʵ�飬��õ��к�����ֵ__(������������С��������������)56.848��