��Ŀ����
����Ŀ��
ͭ���仯�������������������Ź㷺��Ӧ�á���ش��������⣺
(1)ͭԪ��λ��Ԫ�����ڱ��е�������________�壬����Ԫ�����ڱ���________��Ԫ�أ���̬Cuԭ����___________�ֲ�ͬ�ܼ��ĵ��ӡ�
(2)Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu��1958 kJ��mol-1��INi��1753 kJ��mol-1�� ICu��INi��ԭ����________________________________________________________��
(3)����ͭ��������ͭ��Ϊ���Ӿ��壬���߱Ƚϣ��۵�ϸߵ���������ͭ��ԭ��Ϊ_________________________________________��
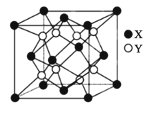
(4)ij��ͭ����������ӽṹ��ͼ1��ʾ��

�ٸ������д��ڵ���������______________(����)��
A�����Ӽ�����B�����ۼ�����C����λ������D����� E�����»���
�ڸ�������̼ԭ�ӵ��ӻ�������____________��
(5)CuCl2��CuCl��ͭ�������Ȼ��
��ͼ2�б�ʾ����________(�CuCl2����CuCl��)�ľ�����
��ԭ�����������������ʾ�����ڲ���ԭ�ӵ����λ�á�ͼ2�и�ԭ���������AΪ(0��0��0)��BΪ(0��1��1)��CΪ(1��1��0)����Dԭ�ӵ��������Ϊ____________��
����֪ͼ2��ʾ������C��D��ԭ�Ӻ˼��Ϊ298pm�������ӵ�������ֵΪNA����þ����ܶ�Ϊ___________________ g��cm��3(�г�����ʽ����)��
���𰸡���B ds 7(����) ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���� O2���뾶��S2���뾶С���������ӵĺ˼��С�������ܴ��۵�� BC sp2��sp3 CuCl (![]() ��
��![]() ��
��![]() )
) 
��������
(1)ͭԪ�������ڱ��е�λ���ǵ�4������B�壬 Cu�ļ۵����Ų�ʽΪ1s22s22p63s23p63d104s1���ݴ˷�����
(2)Cuʧȥһ�����Ӻ���3d�ܼ�ȫ�����Ƚ��ȶ�����ʱʧȥ�ڶ������Ӳ����ף��ݴ˷���ԭ��
(3)�������Ԫ�����Ӱ뾶��С�Ծ����ܵ�Ӱ����з�����
(4)�ٸ���ͼʾ�ɵø������д��ڵ���������
�ڸ���̼ԭ�ӵ����ӷ�ʽ�ж��ӻ���ʽ��
(5)�ٸ��ݾ�����Cu��Cl��ԭ�Ӹ����Ƚ����жϣ�
�ڸ��ݸ�ԭ�ӵĿռ�ṹ�ж�Dԭ�ӵ����������
�۸���![]() ���㾧���ܶȡ�
���㾧���ܶȡ�
(1)ͭԪ�������ڱ��е�λ���ǵ�4������B�壬����Ԫ�����ڱ���ds��Ԫ�أ�Cu�ļ۵����Ų�ʽΪ1s22s22p63s23p63d104s1������7�ֲ�ͬ�ܼ��ĵ��ӣ�
(2)ͭʧȥ����ȫ������3d10���ӣ���ʱʧȥ�ڶ������Ӳ����ף���ʧȥ����4s1���ӣ���ICu��INi��
(3)O2���뾶��S2���뾶С���������ӵĺ˼��С�������ܴ�������ͭ�۵�ߣ�
(4)�ٸ���ͼ1�ɵã��������д��ڷǽ���ԭ��֮��Ĺ��ۼ���Cu2+������ԭ�ӣ�N��Ҳ�γ�����λ�������������Ӽ�������ͷ��Ӽ���������
���Ե������ӵ�̼ԭ���ӻ���ʽΪsp3�ӻ���̼̼˫�����˵�̼ԭ���ӻ���ʽΪsp2�ӻ���
(5)�ٸ��ݾ���ʾ��ͼ��֪��һ�������к���4��Cuԭ�ӣ�Clԭ�Ӹ���Ϊ8��![]() +6��
+6��![]() =4��Cu��Cl��ԭ�Ӹ�����Ϊ1��1���ʸþ���ΪCuCl��
=4��Cu��Cl��ԭ�Ӹ�����Ϊ1��1���ʸþ���ΪCuCl��
��D��C�����ߴ��ھ�����Խ����ϣ���DC���ȵ�����Խ��߳��ȵ�![]() ��D�ڵ���ͶӰD��������Խ���AC�ϣ���AD�����ȵ���D��C���ȵ���������D������(������ϵxOy��)�ľ�����ھ����ⳤ��
��D�ڵ���ͶӰD��������Խ���AC�ϣ���AD�����ȵ���D��C���ȵ���������D������(������ϵxOy��)�ľ�����ھ����ⳤ��![]() ��������z��
��������z��![]() ��D�����ƽ��(������ϵyOz��)�ľ�����ھ����ⳤ��
��D�����ƽ��(������ϵyOz��)�ľ�����ھ����ⳤ��![]() ��������x��
��������x��![]() ��D��ǰƽ��(������ϵxOz��)�ľ�����ھ����ⳤ��
��D��ǰƽ��(������ϵxOz��)�ľ�����ھ����ⳤ��![]() ��������y��
��������y��![]() ����D�����������(
����D�����������(![]() ��
��![]() ��
��![]() )��
)��
�۾�����C��D��ԭ�Ӻ˼��Ϊ298pm������Խ��߳���Ϊ4��298pm��������Խ��߳��ȵ��ھ����ⳤ��![]() ��������������4��
��������������4��![]() g�������ܶȣ�(4��
g�������ܶȣ�(4��![]() g)��(
g)��(![]() cm)3��
cm)3�� ��
��

����Ŀ����ˮ����þ��MgSO4��7H2O����ӡȾ����ֽ��ҽҩ�ȹ�ҵ������Ҫ����;����þ������þ��������ɰ�ķ���������Ҫ�ɷ���MgCO3��������MgO��CaO��Fe2O3��FeO��MnO2��Al2O3��SiO2�����ʣ���ҵ������þ����ȡ��ˮ����þ�Ĺ���������ͼ��

��֪����MnO2������ϡ���ᡣ
��CaSO4��MgSO4��7H2O�ڲ�ͬ�¶��µ��ܽ�ȣ�g���������±���ʾ��
�¶�/�� ���� | 10 | 30 | 40 | 50 | 60 |
CaSO4 | 0.19 | 0.21 | 0.21 | 0.21 | 0.19 |
MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | ���� |
��1����ʼ�õ��������������Ϊ70%���ܶ�Ϊ1.61g/cm3�����������Һ�����ʵ���Ũ��Ϊ___��
��2������A�г�������CaSO4��2H2O�⣬����___��
��3������MgO������е�Ŀ����___��
��4��������B����Ҫ�ɷ�ΪAl(OH)3��Fe(OH)3�������NaClO����������ԭ��Ӧ�����ӷ���ʽΪ___��
��5�������в���1Ϊ����Ũ�������ȹ��ˣ��������ɵõ�CaSO4��2H2O���ַ�ֹ___��
��6����ȡMgSO4��7H2O�IJ���2Ϊ��___��___������ϴ�ӡ�
��7����֪��ʼ��þ����Ʒ������Ϊag����ȡ��ˮ����þ������Ϊbg���ݴ��ܼ������þ����þԪ�صĺ��������ܣ���д������ʽ�������ܣ���˵�����ɡ�___���ܻ��ܣ�������ʽ�������ɣ�Ϊ___��