��Ŀ����
�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
| �ɷ� | ������g�� | Ħ��������g?mol-1�� |
| ���� | 25.0 | 342 |
| ����� | 0.435 | 174 |
| ��˾ƥ�� | 0.4 | 180 |
| ������� | 0.237 | 158 |
| ������ | 0.2 | 170 |
A�������������� B��������ء����� C������ء��� D������
��2�����ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ______mol?L-1��
��3��������һ�����ʵ���Ũ�ȵ���Һʱ���ɷ�Ϊ���¼������裺�ٳ����ڼ��� ���ܽ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳��Ϊ______��
��4����������500mL���ʻ����ʼ�������������У�������ƽ���ձ�����������ҩ�ס�______��______�����ں�������д��ȱ���������ƣ���
��5������Һ���ƹ����У����в������K+�����ʵ���Ũ��ƫ�ߵ���______��
A������ʱ���ӿ̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�KCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ�����
�⣺��1����������������������ǵ���ʣ�����ǿ�����ԣ������Ƿǵ���ʣ� ��������ǵ���ʣ���ǿ�����ԣ�
��ѡ��B��
��2��������ص����ʵ���Ϊn��KMnO4��= =
=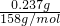 =0.0015mol��
=0.0015mol��
����ص����ʵ���Ϊn��K2SO4��=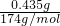 =0.0025mol��
=0.0025mol��
n��K+��=n��KMnO4��+2n��K2SO4��=0.0015mol+2��0.0025mol=0.0065mol��
����c��K+��=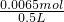 =0.013mol/L��
=0.013mol/L��
�ʴ�Ϊ��0.013mol/L��
��3�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢݢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢݢߢܣ�
��4���ɣ�3���в�����֪��������500mL��Һ������ѡ��500mL����ƿ������轺ͷ�ιܶ��ݣ���������������������ƽ���ձ�����������ҩ�����500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��4��A������ʱ���ӿ̶��ߣ�������Һ���ƫС��������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�KCl��Һ��δϴ��������ƿմ���Ȼ��أ��Ȼ��ص�ʵ������ƫ��������ҺŨ��ƫ�ߣ�
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬δ���κδ�������������ҺŨ����Ӱ�죮
��ѡ��AC��
��������1�������֪����Һ�л�����״̬���ܵ���Ļ����������ؾ���ǿ�����ԣ�
��2��K+���Ը�����ء�����صĵ��룬��n= ���������ء�����ص����ʵ�����
���������ء�����ص����ʵ�����
���ݼ������غ��֪n��K+��=n��KMnO4��+2n��K2SO4�����ٸ������ʵ���Ũ�ȶ��������������ʵ���Ũ�ȣ�
��3������������Һ��ʵ��������̽���ʵ�鲽������
��4��������Һ�����Ʋ���������ʹ�õ�������
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c= �����жϣ�
�����жϣ�
���������ʻ����ʼ�Ϊ���壬��Ҫ������Һ���ƣ��Ѷ��еȣ�ע���c= ��������ԭ����
��������ԭ����
��ѡ��B��
��2��������ص����ʵ���Ϊn��KMnO4��=
 =
=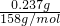 =0.0015mol��
=0.0015mol������ص����ʵ���Ϊn��K2SO4��=
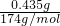 =0.0025mol��
=0.0025mol��n��K+��=n��KMnO4��+2n��K2SO4��=0.0015mol+2��0.0025mol=0.0065mol��
����c��K+��=
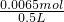 =0.013mol/L��
=0.013mol/L���ʴ�Ϊ��0.013mol/L��
��3�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢݢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢݢߢܣ�
��4���ɣ�3���в�����֪��������500mL��Һ������ѡ��500mL����ƿ������轺ͷ�ιܶ��ݣ���������������������ƽ���ձ�����������ҩ�����500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��4��A������ʱ���ӿ̶��ߣ�������Һ���ƫС��������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�KCl��Һ��δϴ��������ƿմ���Ȼ��أ��Ȼ��ص�ʵ������ƫ��������ҺŨ��ƫ�ߣ�
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬δ���κδ�������������ҺŨ����Ӱ�죮
��ѡ��AC��
��������1�������֪����Һ�л�����״̬���ܵ���Ļ����������ؾ���ǿ�����ԣ�
��2��K+���Ը�����ء�����صĵ��룬��n=
 ���������ء�����ص����ʵ�����
���������ء�����ص����ʵ��������ݼ������غ��֪n��K+��=n��KMnO4��+2n��K2SO4�����ٸ������ʵ���Ũ�ȶ��������������ʵ���Ũ�ȣ�
��3������������Һ��ʵ��������̽���ʵ�鲽������
��4��������Һ�����Ʋ���������ʹ�õ�������
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
 �����жϣ�
�����жϣ����������ʻ����ʼ�Ϊ���壬��Ҫ������Һ���ƣ��Ѷ��еȣ�ע���c=
 ��������ԭ����
��������ԭ���� 
��ϰ��ϵ�д�
�����Ŀ
 �ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺