��Ŀ����
��֪��Ԫ��H2A�ĵ��뷽��ʽΪ��H2A=H++HA- HA-?H++A2-
��������ش��������⣺
��1��NaHA��Һ��
��2����0.01mol/L��NaHA��Һ��c��A2-��=amol/L����0.01mol/L��H2A��Һ��c��A2-��
��3��ij��Һ��1mol/LNaHA��Һ��1mol/LNaOH��Һ�ڳ����µ������϶��ã������Һ��pH
��4������A��B��C��D������Һ�����Ƿֱ���Na2A��Һ�����ᡢ��ˮ��Na2SO4��Һ�е�һ�֣���֪A��B��Һ��ˮ�ĵ���̶���ͬ��A��C��Һ��pH��ͬ����A��Һ��
��������ش��������⣺
��1��NaHA��Һ��
��
��
�ԣ���ᡱ��������С�����NaHA��Һ�д��ڵķ�����H2O
H2O
��2����0.01mol/L��NaHA��Һ��c��A2-��=amol/L����0.01mol/L��H2A��Һ��c��A2-��
��
��
amol/L�����������������=�������ж�������H2A��һ���������ɵ�H+������HA-�ĵ���
H2A��һ���������ɵ�H+������HA-�ĵ���
����3��ij��Һ��1mol/LNaHA��Һ��1mol/LNaOH��Һ�ڳ����µ������϶��ã������Һ��pH
��
��
7 �����������������=��������Һ������Ũ���ɴ�С˳��Ϊc��Na+����c��A2-����c��OH-����c��HA-����c��H+��
c��Na+����c��A2-����c��OH-����c��HA-����c��H+��
����4������A��B��C��D������Һ�����Ƿֱ���Na2A��Һ�����ᡢ��ˮ��Na2SO4��Һ�е�һ�֣���֪A��B��Һ��ˮ�ĵ���̶���ͬ��A��C��Һ��pH��ͬ����A��Һ��
��ˮ
��ˮ
��������Һ��ˮ�ĵ���̶��ɴ�С�Ĺ�ϵΪC��D��A=B
C��D��A=B
�����á�A��B��C��D����ʾ����������1���ɵ�һ����ȫ���룬��NaHA��Һ����Ҫ����HA-�ĵ��룬����HA-?A2-+H+����Һ�����ԣ�����HA-��ˮ�⣬����û��H2A������NaHA��Һ�д��ڵķ���ֻ��H2O��
��2������H2A=H++HA-���ɵ�H+�����˵ڶ������룬��H2A��Һ��HA-?H++A2-�������ɵ�A2-С��NaHA��Һ��c��A2-����
��3��1mol/LNaHA��Һ��1mol/LNaOH��Һ��Ӧ����Na2A��Һ��Na2Aˮ���Լ��ԣ��ٸ�����Һ��ˮ�ⷽ�̺͵��뷽��ʽ�жϵ����Ӵ�Сд�������ӵĹ�ϵ��
��4����֪A��B��Һ��ˮ�ĵ���̶���ͬ�����ᡢ��ˮ��������ˮ�ĵ��룬A��C��Һ��pH��ͬ��Na2A��Һ����ˮ�Լ��ԣ���AΪ��ˮ��CΪNa2A��Һ��BΪ���DΪNa2SO4��
��2������H2A=H++HA-���ɵ�H+�����˵ڶ������룬��H2A��Һ��HA-?H++A2-�������ɵ�A2-С��NaHA��Һ��c��A2-����
��3��1mol/LNaHA��Һ��1mol/LNaOH��Һ��Ӧ����Na2A��Һ��Na2Aˮ���Լ��ԣ��ٸ�����Һ��ˮ�ⷽ�̺͵��뷽��ʽ�жϵ����Ӵ�Сд�������ӵĹ�ϵ��
��4����֪A��B��Һ��ˮ�ĵ���̶���ͬ�����ᡢ��ˮ��������ˮ�ĵ��룬A��C��Һ��pH��ͬ��Na2A��Һ����ˮ�Լ��ԣ���AΪ��ˮ��CΪNa2A��Һ��BΪ���DΪNa2SO4��
����⣺��1���ɵ�һ����ȫ���룬��NaHA��Һ����Ҫ����HA-�ĵ��룬����HA-?A2-+H+����Һ�����ԣ�����HA-��ˮ�⣬����û��H2A������NaHA��Һ�д��ڵķ���ֻ��H2O���ʴ�Ϊ���H2O��
��2����0.01mol/L��NaHA��Һ��c��A2-��=amol/L����0.01mol/L��H2A��Һ����һ���������ɵ�H+������HA-�ĵ��룬������Һ��A2-�����ʵ���Ũ��С��С������c��A2-����amol/L���ʴ�Ϊ������H2A��һ���������ɵ�H+������HA-�ĵ��룻
��3��1mol/LNaHA��Һ��1mol/LNaOH��Һ��Ӧ����Na2A��Һ��Na2Aˮ���Լ��ԣ�����pH��7����Һ�д���ˮ�ⷽ��A2-+H2O?HA-+OH-�����뷽��H2O?H++OH-����c��OH-����c��H+��������c��Na+����c��A2-����c��OH-����c��HA-����c��H+�����ʴ�Ϊ������c��Na+����c��A2-����c��OH-����c��HA-����c��H+����
��4����֪A��B��Һ��ˮ�ĵ���̶���ͬ�����ᡢ��ˮ��������ˮ�ĵ��룬A��C��Һ��pH��ͬ��Na2A��Һ����ˮ�Լ��ԣ���AΪ��ˮ��CΪNa2A��Һ��BΪ���DΪNa2SO4�����ᡢ��ˮ��������ˮ�ĵ��룬Na2A��Һ�ٽ�ˮ�ĵ��룬Na2SO4��ˮ�ĵ�����Ӱ�죻����������Һ��ˮ�ĵ���̶��ɴ�С�Ĺ�ϵΪ��C��D��A=B��
�ʴ�Ϊ����ˮ��C��D��A=B��
��2����0.01mol/L��NaHA��Һ��c��A2-��=amol/L����0.01mol/L��H2A��Һ����һ���������ɵ�H+������HA-�ĵ��룬������Һ��A2-�����ʵ���Ũ��С��С������c��A2-����amol/L���ʴ�Ϊ������H2A��һ���������ɵ�H+������HA-�ĵ��룻
��3��1mol/LNaHA��Һ��1mol/LNaOH��Һ��Ӧ����Na2A��Һ��Na2Aˮ���Լ��ԣ�����pH��7����Һ�д���ˮ�ⷽ��A2-+H2O?HA-+OH-�����뷽��H2O?H++OH-����c��OH-����c��H+��������c��Na+����c��A2-����c��OH-����c��HA-����c��H+�����ʴ�Ϊ������c��Na+����c��A2-����c��OH-����c��HA-����c��H+����
��4����֪A��B��Һ��ˮ�ĵ���̶���ͬ�����ᡢ��ˮ��������ˮ�ĵ��룬A��C��Һ��pH��ͬ��Na2A��Һ����ˮ�Լ��ԣ���AΪ��ˮ��CΪNa2A��Һ��BΪ���DΪNa2SO4�����ᡢ��ˮ��������ˮ�ĵ��룬Na2A��Һ�ٽ�ˮ�ĵ��룬Na2SO4��ˮ�ĵ�����Ӱ�죻����������Һ��ˮ�ĵ���̶��ɴ�С�Ĺ�ϵΪ��C��D��A=B��
�ʴ�Ϊ����ˮ��C��D��A=B��
���������⿼������ˮ�⼰����Ũ�ȴ�С�ıȽϣ�Ӱ��ˮ��������أ�ˮ��Һ������Ũ�ȵ����㣬��ȷϰ���е���Ϣ�ǽ����Ĺؼ���ע���Ԫ������������ص㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
�о���ѧ��Ӧԭ������������������壬���û�ѧ��Ӧԭ�������֪ʶ�ش��������⣺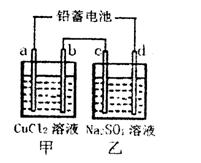
��1����Ǧ���ص��ס����������е���Һ����֪Ǧ���ص��ܷ�ӦΪ��Pb��s����PbO2��s����2H2SO4��aq��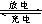 2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����______
2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����______ ______
______
����д��ţ�
| A��d������ |
| B�������ü׳ؾ���ͭ��b��ӦΪ��ͭ |
| C���ŵ�ʱǦ���ظ����ĵ缫��ӦʽΪ�� PbO2��s����4H����aq���� 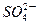 ��aq����4e�� ��aq����4e�� PbSO4��s����2H2O��1�� PbSO4��s����2H2O��1�� |
| D�����ĸ��缫���Ͼ�Ϊʯī��������6��4g Cuʱ�������й���������3��36L���� |
��2��ij��Ԫ��H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A��H����HA����HA��
 H����A2������ش��������⣺
H����A2������ش��������⣺��Na2A��Һ��_________������ԡ��������ԡ����ԡ�����������________________
�������ӷ���ʽ��ʾ����
����֪0��1
 mol��L��1��NaHA��Һ��pH��2����0��1mol��L��1��H2A��Һ�������ӵ����ʵ���Ũ�ȿ�����__________0��1lmol��L��1���<������>���������������ǣ�___________________________.
mol��L��1��NaHA��Һ��pH��2����0��1mol��L��1��H2A��Һ�������ӵ����ʵ���Ũ�ȿ�����__________0��1lmol��L��1���<������>���������������ǣ�___________________________.��3����������һ����Ҫ�����ȼ�ϣ�����ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g����CO��g��
 CH3OH��g���� ��H����90��8kJ��mol��1
CH3OH��g���� ��H����90��8kJ��mol��1��2
 CH3OH��g��
CH3OH��g�� CH3OCH3��g����H2O��g���� ��H����23��5kJ��mol��1
CH3OCH3��g����H2O��g���� ��H����23��5kJ��mol��1��CO��g����H2O��g��
 CO2��g����H2��g����������41��3kJ��mol��1
CO2��g����H2��g����������41��3kJ��mol��1д��ˮú��ֱ�Ӻϳɶ�����ͬʱ����CO2���Ȼ�ѧ��Ӧ����ʽ___________________.
��4��SO2��������������Ҫ�м���Ҳ�ǿ�����Ⱦ����Ҫԭ��֮һ������������SO3�ķ�ӦΪ��2SO2��g����O2��g��
 2SO3��g������һ���¶��£���0��23 mol SO2��0��11 mol���������ݻ�Ϊl L���ܱ������з�����Ӧ���ﵽƽ���õ�0��12 mol SO3����Ӧ��ƽ�ⳣ��K��________�����¶Ȳ��䣬�ټ���0��50 mol���������´ﵽƽ�⣬��SO3�����������___________������������䡱��С������
2SO3��g������һ���¶��£���0��23 mol SO2��0��11 mol���������ݻ�Ϊl L���ܱ������з�����Ӧ���ﵽƽ���õ�0��12 mol SO3����Ӧ��ƽ�ⳣ��K��________�����¶Ȳ��䣬�ټ���0��50 mol���������´ﵽƽ�⣬��SO3�����������___________������������䡱��С������ 
 2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________
2PbSO4��s����2H2O��1�������һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________ ��aq����4e��
��aq����4e�� PbSO4��s����2H2O��1��
PbSO4��s����2H2O��1�� H����A2������ش��������⣺
H����A2������ش��������⣺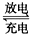 2PbSO4(s) +2H2O(l)�����һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________����д��ţ���
2PbSO4(s) +2H2O(l)�����һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ����____________����д��ţ��� 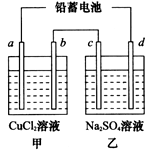
 H++A2-��
H++A2-��  CH3OH(g) ��H=-90.8kJ/mol
CH3OH(g) ��H=-90.8kJ/mol  CH3OCH3(g)+H2O(g) ��H=-23.5kJ/mol
CH3OCH3(g)+H2O(g) ��H=-23.5kJ/mol  CO2(g)+H2(g) ��H=-41.3kJ/mol
CO2(g)+H2(g) ��H=-41.3kJ/mol  2SO3(g)����һ���¶��£���0.23molSO2��0.11molO2ͨ���ݻ�Ϊ1L���ܱ������з�����Ӧ���ﵽƽ���õ�0.12molSO3����Ӧ��ƽ�ⳣ��K=__________�����¶Ȳ��䣬��ͨ��0.50molO2�����´ﵽƽ�⣬��SO3�����������__________����������䡱��С������
2SO3(g)����һ���¶��£���0.23molSO2��0.11molO2ͨ���ݻ�Ϊ1L���ܱ������з�����Ӧ���ﵽƽ���õ�0.12molSO3����Ӧ��ƽ�ⳣ��K=__________�����¶Ȳ��䣬��ͨ��0.50molO2�����´ﵽƽ�⣬��SO3�����������__________����������䡱��С������