��Ŀ����
����Ŀ����Ԫ�����ڱ��У���ϡ�������⼸������Ԫ�ض��������γ��⻯�
(1)�������ڹ������⻯���ҵ���ð����ʹ��������ͭ��Cu(NH3)2]Ac�Ļ��Һ������CO����������Ӽ�дΪAc-������Ӧ����ʽΪ����Cu(NH3)2]Ac+CO+NH3=[Cu(NH3)3CO]Ac
�ٰ�ˮ��Һ�и�Ԫ��ԭ�ӵĵ�һ�����ܴӴ�С����˳��Ϊ___________��
�ڴ������(CH3COOH)�е�����̼ԭ�ӵ��ӻ���ʽ�ֱ���_________________��
��������[Cu(NH3)3CO]Ac��������ѧ��������_________������ţ���
A�����Ӽ�b��������c�����ۼ�d����λ��
(2)ij���ӻ�����XY2�������ṹ��ͼ��ʾ������6��Yԭ��������1��6��ע��
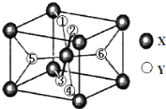
��֪1��2��3��4��Yԭ���ھ����ϡ������ϣ���5��6��Yԭ�Ӿ��ھ���_________������桱���ڲ�������
�ڸ���������Ϣ������֪��XY2������۷е�______���>������=����<������̬�����۷е㡣
�����þ����ı߳�Ϊanm���ܶ�Ϊ��g/cm3��XY2��Ħ������ΪMg/mol�����ӵ�����Ϊ____��
���𰸡�N��O��H sp3 sp2 acd �ڲ� > ![]()
��������
(1)��ͬ���ڣ���һ�����ܾ�����������ƣ�����VA����ڵ�VIA�壻��CH3COOH�еĵ�һ��̼ԭ���γ�4�����ۼ����ڶ���̼ԭ���γ�3�����ۼ�����������[Cu(NH3)3CO]Ac��Ac����[Cu(NH3)3CO]2+�γ����Ӽ���NH3���й��ۼ���[Cu(NH3)3CO]2+������λ����
(2)��X���Ӹ���Ϊ2�������ݻ�ѧʽXY2�����Y���Ӹ���Ϊ4�����������ļ��������ȷ����������Y��λ�ã��ڸ���������Ϣ������֪��XY2�����Ӿ��壬��̬���Ƿ��Ӿ��壻�۸þ���X���Ӹ���Ϊ2����Y���Ӹ���Ϊ4��������![]() ������ϵʽ���㰢���ӵ�������
������ϵʽ���㰢���ӵ�������
(1)�ٰ�ˮ��Һ��Ԫ��ΪH��N��Oԭ�ӵĵ�һ�����ܴӴ�С����˳��ΪN��O��H���ʴ�Ϊ��N��O��H��
�ڴ������(CH3COOH)�еĵ�һ��̼ԭ���γ�4�����ۼ�����4���������ӻ���ʽΪsp3���ڶ���̼ԭ���γ�3�����ۼ�����3��������û��δ�ɶԵ��ӣ��ӻ���ʽΪsp2���ʴ�Ϊ��sp3��sp2��
��������[Cu(NH3)3CO]Ac��Ac����[Cu(NH3)3CO]2+�γ����Ӽ���NH3���й��ۼ���[Cu(NH3)3CO]2+������λ���������������������ѧ��������abd���ʴ�Ϊ��abd��
(2)��X���Ӹ���Ϊ2�������ݻ�ѧʽXY2�����Y���Ӹ���Ϊ4������֪1��2��3��4��Yԭ���ھ����ϡ������ϣ�����2��������2��Y������5��6�ţ����5��6��Yԭ�Ӿ��ھ����ڲ����ʴ�Ϊ���ڲ���
�ڸ���������Ϣ������֪��XY2�����Ӿ��壬��̬���Ƿ��Ӿ��壬���XY2������۷е㣾��̬�����۷е㣻�ʴ�Ϊ������
�����þ���X���Ӹ���Ϊ2����Y���Ӹ���Ϊ4�������� �����ӵ�����Ϊ
�����ӵ�����Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

����Ŀ���о�����ȿ���CO��CO2��Ӧ�öԹ�����̬������������Ҫ�����塣
��1��CO��������������֪��Fe2O3��s��+ 3C��s����2Fe��s��+ 3CO��g�� ��H 1��+489.0 kJ��mol��1
C��s�� +CO2��g����2CO��g�� ��H 2 ��+172.5 kJ��mol��1����CO��ԭFe2O3��s�����Ȼ�ѧ����ʽΪ_________________________________________________��
��2�������¯ú���õ���CO���������Ƴ�ȼ�ϵ�أ���KOH��ҺΪ���Һ����д���õ�صĸ�����Ӧʽ��__________________________________________________��
��3����CO2��H2����һ��������ܱ������У��������¶��·�����Ӧ��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g�������CH3OH�����ʵ�����ʱ��ı仯��ͼ��������I�����Ӧ��ƽ�ⳣ����С��ϵΪK��___________________K��������������������������������
CH3OH��g��+H2O��g�������CH3OH�����ʵ�����ʱ��ı仯��ͼ��������I�����Ӧ��ƽ�ⳣ����С��ϵΪK��___________________K��������������������������������

��һ���¶��£����ݻ���ͬ�ҹ̶��������ܱ������У������·�ʽ���뷴Ӧ�һ��ʱ���ﵽƽ�⡣
�� �� | �� | �� |
��Ӧ��Ͷ���� | 1molCO2��3molH2 | a molCO2��b molH2�� c molCH3OH��g����c molH2O��g�� |
������ƽ��������ѹǿΪ��ʼ��0.8����Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У���c��ȡֵ��ΧΪ______________________��
��һ���¶��£��˷�Ӧ����ѹ�����н��У����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������______________��
a��������ѹǿ���� b��H2������������� c��c��H2����3c��CH3OH��
d���������ܶȲ��� e��2��C��O���ѵ�ͬʱ��3��H��H����
��4����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2��g�� + 6H2��g��![]() CH3OCH3��g�� + 3H2O��g������֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯������ͼ�����¶Ȳ��䣬���Ͷ�ϱ�n(H2)/n(CO2)����K��__________���÷�Ӧ��H_________0����������������������=������
CH3OCH3��g�� + 3H2O��g������֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯������ͼ�����¶Ȳ��䣬���Ͷ�ϱ�n(H2)/n(CO2)����K��__________���÷�Ӧ��H_________0����������������������=������



