��Ŀ����
��1����Һ��������Ũ���ɴ�С��ϵ��
��2����Һ����c��CO32-��
��3���ס�����Һ�������ӵ����ʵ���Ũ��֮�͵Ĵ�С��ϵ����Һ��
II���ں㶨����������£�����0.1molNaCl��0.1molCuSO4�Ļ����Һ500mL�����е�⣬2min��������ʼ�������壬5minʱֹͣ��⣬��ʱ�������������������������ͬ��
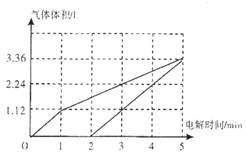
��1������ͼ�л��������������������������ѻ��ɱ�״���µ��������ʱ��Ĺ�ϵ��
��2��������ʱ����ˮ������Ϊ
��2��NaHCO3��Һ�У��Լ��ԣ�HCO3-��ˮ���������룻
��3��Na2CO3��Һ��CO32-��ˮ���������HCO3-��ˮ�⣻
II����Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-��
��������2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O����������Cu2++2e-�TCu��2H++2e-�TH2�����������ӷŵ�ʵ��Ϊ���ˮ��������Һ��������NaOH�����ʵ�����ͬ�������ӹ������Դ˼��㣮
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��2��NaHCO3��Һ�У��Լ��ԣ�HCO3-��ˮ���������룬ˮ������̼�ᣬ��c��CO32-����c��H2CO3���ʴ�Ϊ������
��3��Na2CO3��Һ��CO32-��ˮ���������HCO3-��ˮ�⣬�����������Ũ��֮�ʹ����Լף��ң��ʴ�Ϊ������
II����Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-��
2min֮ǰ����������2Cl--2e-�TCl2����
0.1mol 0.1 0.05
��������Cu2++2e-�TCu
0.05 0.1
2min��5min��
��������4OH--4e-�TO2��+H2O
4x x
��������Cu2++2e-�TCu
0.05 0.1
2H++2e-�TH2��
4x-0.1 2x-0.05��
�������������������������ͬ����0.05+x=2x-0.05�����x=0.1�����������ռ�0.15mol���壬
��ͼ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2���������ӷŵ�ʵ��Ϊ���ˮ����2H2O
| ||
������0.1mol������������ˮΪ0.2mol��������Ϊ0.2mol��18g/mol=3.6g��
������0.1molNaHSO4��ϡ����1000mL����c��H+��=
| 0.1mol |
| 1L |

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д���13�֣�����Ũ��Ϊ0.1 mol?L��1�����ֵ������Һ�� �� Na2CO3 �� NaHCO3 �� NaAlO2 �� CH3COONa �� NaOH
��֪��CO2+3H2O+2AlO2��===2Al(OH)3��+CO32��
��1����������Һ��pH��С�����˳����___ ___�����ţ���
��2����������Һϡ����ͬ�ı���ʱ����pH�仯������____ __�����ţ���
��3�����̼�ᣨH2CO3����Һ��NaAlO2��Һ����д�����ܷ����Ļ�ѧ��Ӧ����ʽ��
��
��4�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
�� | 0.20 | 0.20 | pH��a |
�� | 0.10 | 0.10 | pH��8.00 |
�����������ʵ���������Ӽ�����������������a�������Һ��pH����˵��HA��ǿ�ỹ������ ��
����ʵ�����û����Һ����ˮ�������c (OH��)�� mol/L��
����û����Һ��������ʽ��ֵ��Ҫ��д��������������̡�
I��c (Na��)��c (A��)�� ��
��c (OH��)��c (HA)�� ��
��1����������Һ��pH��С�����˳����______�����ţ���
��2����������Һϡ����ͬ�ı���ʱ����pH�仯������______�����ţ���
��3�����̼�ᣨH2CO3����Һ��NaAlO2��Һ����д�����ܷ����Ļ�ѧ��Ӧ����ʽ��______��
��4�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.20 | 0.20 | pH=a |
| �� | 0.10 | 0.10 | pH=8.00 |
����ʵ�����û����Һ����ˮ�������c ��OH-��=______mol/L��
����û����Һ��������ʽ��ֵ��
I��c ��Na+��-c ��A-��=______��
II��c ��OH-��-c ��HA��=______��