��Ŀ����
����˵�����ʾ������ȷ����
[ ]
A����Ҫ���ȷ����ķ�Ӧ�����ȷ�Ӧ
B����ϡ��Һ�У�H��(aq)��OH��(aq)��H2O(l)����H����57.3kJ/mol��������0.5mol H2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ
C����C(ʯī)��C(���ʯ)����H����1.73 kJ/mol����֪���ʯ��ʯī�ȶ�
D����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)��O2(g)��2H2O(l)����H����285.8kJ/mol
B����ϡ��Һ�У�H��(aq)��OH��(aq)��H2O(l)����H����57.3kJ/mol��������0.5mol H2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ
C����C(ʯī)��C(���ʯ)����H����1.73 kJ/mol����֪���ʯ��ʯī�ȶ�
D����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)��O2(g)��2H2O(l)����H����285.8kJ/mol
B

��ϰ��ϵ�д�
�����Ŀ
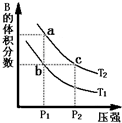 ���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮

