��Ŀ����
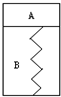
��ͼ��ʾ��ij������������һ�����͵��ɣ�B��Ϊ��գ�A���ѳ���3mol H2��1 mol N2���������淴Ӧ��3H2(S)+N2(g)
(1)��A��Ѹ���ٳ���3molH2��1 molN2������������˶�?_________________________���������ٴα��־�ֹʱ��H2�ķ�Ӧ����v��ԭƽ��ʱH2�ķ�Ӧ����v0�Ĵ�С��ϵΪv________v0(�������������=��)��ͬʱH2��ת���ʱ�ԭƽ��ʱ________(�������С�����䡱)��A���������________(�������������=��)ԭA���������2����
(2)������������ȣ�ʹA�������¶����ߣ������������ƶ��������»���ԭ���ǣ�
��__________________________________��
��__________________________________��
(1)�����������˶������������˶� �� ���� �� (2)�ٸ���ƽ���ƶ�ԭ��������ƽ�����淴Ӧ�����ƶ�������������ʵ������Ӷ�ѹǿ���������¶ȣ����屾���������ͣ��Ӷ�ѹǿ����
�����������黯ѧƽ�⣬�����⡣(1)Ѹ�ٳ���3molH2��1 mol N2����ʼʱѹǿ�������������ƶ�������ѹǿ����ƽ��������Ӧ�����ƶ���������������С�������������ƶ���(2)�����¶ȣ�ƽ�����淴Ӧ�����ƶ�����������ʵ������࣬����ѹǿ�����������ƶ���

 ��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ�������
��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ������� ��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ�������
��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ�������
