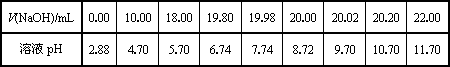��Ŀ����
ijʵ��С����������к͵ζ����ⶨʳ����������g/100mL��������������뱾ʵ�鲢�ش�������⣮���й�ʵ��ҩƷΪ������ʳ�ð״���Ʒ500mL��0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��0.1%ʯ����Һ����
��ʵ�鲽�裺
��1���õζ�����ȡ10mL���۰״���Ʒ������100mL����ƿ�У�������ˮ����г�ȥCO2��Ѹ����ȴ����ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
��2������ʽ�ζ���ȡ����ʳ����Һ20.00mL��______�У�
��3��ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
��4���ζ�������¼NaOH���ն������ظ��ζ�2-3�Σ�
��ʵ���¼�����ݴ���
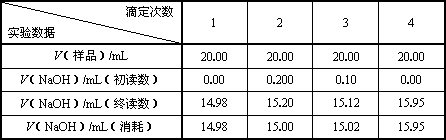
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
���������ۣ�
��1����ͬѧ�ڴ������ݹ����м���ã�V��NaOH����ƽ�����ģ�=1/4��14.98+15.00+15.02+15.95��mL=15.24mL��
�Է������ļ����Ƿ�����������������˵�����ɣ�______��
��2����ͬѧ��0.1000mol/LNaOH��Һ�ζ���һ���۰״���Ʒ��Һʱ���ζ�������ʹ��pH�ƽ���Һ��pH�仯�����¼���±���
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |
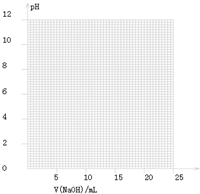
���ɱ���ͼ��֪������������Χ����0.1%���ڣ�pHͻ�䣨�ζ�ͻԾ����ΧΪ______�����Կ�ѡ��______��ָʾ����
��������ָʾ���ı�ɫ��Χ
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |
������ʵ�����ݽ�Ϸ�ӦCH3COOH+NaOH=CH3COONa+H2O��ʵ��1��2��3ƽ����������������Һ���=
 =15.00ml��c����Ʒ��V����Ʒ��=c��NaOH��V��NaOH��
=15.00ml��c����Ʒ��V����Ʒ��=c��NaOH��V��NaOH��c����Ʒ����20.00ml=0.1000mol/L��15.00ml
c����Ʒ��=0.75mol/L��
��Ʒ������g/100mL=
 =4.5g/100ml��
=4.5g/100ml���ʴ�Ϊ��0.75mo/L��4.5g/100ml��
��1����ͬѧ�ڴ������ݹ����еļ��㲻��������Ϊ��4��ʵ���������̫��Ӧ��ȥ��
�ʴ�Ϊ������������Ϊ������������ǰ3�����ϴ����쳣ֵ��Ӧ��ȥ��
��2��������ͼ�����ݰ�����㷨����ͼ��
 ���ʴ�Ϊ��
���ʴ�Ϊ��
������ͼ���ͼ�����ݷ�����֪PHͻ����7.74��9.70���������������ǡ�÷�Ӧ���ɵĴ�������Һ�ʼ��ԣ���̪PH��ɫ��ΧΪ8.2��10.0������ָʾ��ѡ���̪�Ϻõ��жϷ�Ӧ�յ㣻
�ʴ�Ϊ��7.74��9.70����̪��
�������ζ�������Һ�ڵζ����У�����Һʢ����ƿ�У�
����ͼ�����ݽ�ϴ�����������ư���1��1��Ӧ������Ʒ��ҺŨ�ȣ�����Ũ�ȼ�����Ʒ��������
������ͼ����������㷨�����仯���ߣ�
�ڸ��ݻ���ͼ������жϣ�pHͻ�䣨�ζ�ͻԾ��������PHͻ����ָʾ����ɫ��Χѡ����Ҫ��ָʾ����
���������⿼��������к͵ζ�ʵ��IJ�������ͼ���Ӧ�ã���Ӧ�յ��ͼ����ƣ��յ��жϣ�ָʾ����ѡ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȡ���ش�������⣺
��1��Ŀǰ�����һ��������Ʊ��������⣬��Ҫ��������ͼ���˹��̵��ܷ���ʽΪ ��
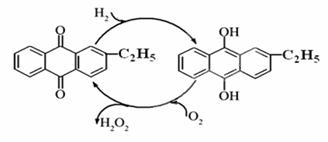
��2��ʵ���ó�������������Ϊ0.51%H2O2ˮ��Һ���ܶ�Ϊ1g/mL����pHΪ5
i.д��H2O2����ˮ�ĵ��뷽��ʽ .
ii.�ⶨH2O2ˮ��ҺpH�ķ���Ϊ(����)
A.�����ָʾ���ⶨ B.�ù㷺pH��ֽ�ⶨ
C.�þ���pH��ֽ�ⶨ D.��pH�Ʋⶨ
��3��ijʵ��С�����о�Ũ�ȡ���������Һ����Զ�H2O2�ֽⷴӦ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10 mL 2% H2O2��Һ | �� |
| �� | 10 mL 5% H2O2��Һ | �� |
| �� | 10 mL 5% H2O2��Һ | 0.1gMnO2��ĩ |
| �� | 10 mL 5% H2O2��Һ������HCl��Һ | 0.1gMnO2��ĩ |
| �� | 10 mL 5% H2O2��Һ������NaOH��Һ | 0.1gMnO2��ĩ |
i.ʵ��ٺ͢ڵ�Ŀ����_______��ʵ��ʱ����û�й۲쵽������������ó����ۡ�������ʾ��ͨ��������H2O2���ȶ������ֽ⡣Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ���_________��
ii.ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
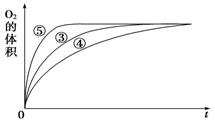
������ͼ�ܹ��ó���ʵ�������____________��
��4��ʵ���ҳ������Ը�����ر���Һ�ⶨ˫��ˮ��Ũ�ȣ���Ӧԭ��Ϊ��
MnO4����H2O2��H�� ��Mn2����H2O�� O2��
i.����ƽ�������ӷ���ʽ
ii.����Һ����ȡ25.00mL����������ƿ�У��ظ��ζ��ĴΣ�ÿ������0.1000 mol��L-1��KMnO4����Һ������±���ʾ��
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| �����mL�� | 17.10 | 18.10 | 18.00 | 17.90 |
���������й��������Ũ��Ϊ mol��L-1��
iii.���ζ�ǰ�����������ݵζ�����ʧ����ⶨ��� ����ƫ�ߡ���ƫ�͡����䡱����