��Ŀ����
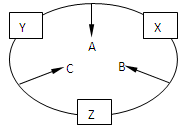
 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��
������C��������һ������ش��������⣺
��1����Ԫ��X��ԭ�ӽṹʾ��ͼ______
��2����C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ______
��3���������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ�������������ӷ���ʽ��ʾ������ԭ��
______
��4����д��A��C��Ӧ����Y��B�Ļ�ѧ����ʽ______
��5�������������£�Cͨ��װ�к�X�ĺ�ɫ��ĩ��Ӳ�ʲ����ܣ���ɫ��ĩ����Ϻ�ɫ���ú�ɫ��ĩ�Ļ�ѧʽ______��������Ӧ��ѧ����ʽΪ______��
�⣺X��Y��Z���ֶ�����Ԫ�أ����ǵ�ԭ������֮��Ϊ16�����������γ���ɫ���嵥�ʵ�ֻ��H2��N2��O2��ϡ��������⣩�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������˵����NH3��Y��ZΪN2��O2�����ת����ϵ�жϣ�X+Z=B��Z+Y=C��X+Y=A��һ��B�����к��е�Zԭ�Ӹ�����C��������1����˵��ZԪ��ΪH����X��Y��Z�ֱ�ΪO��N��H��A��B��C�ֱ�ΪNO��H2O��NH3��
��1��Ԫ��XΪOԪ�أ�ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��C��NH3����X��O2����һ�����������ɻ�����A��NO���Ļ�ѧ����ʽΪ4NH3+5O2 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2 4NO+6H2O��
4NO+6H2O��
��3�������£�C��NH3����ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������������ԭ����NH3?H2O NH4++OH-���ʴ�Ϊ��NH3?H2O
NH4++OH-���ʴ�Ϊ��NH3?H2O NH4++OH-��
NH4++OH-��
��4��A��NO����C��NH3����Ӧ����Y��N2����B��H2O���Ļ�ѧ����ʽΪ��4NH3+6NO�T5N2+6H2O���ʴ�Ϊ��4NH3+6NO�T5N2+6H2O��
��5�����������£�Cͨ��װ�к�X�ĺ�ɫ��ĩ��Ӳ�ʲ����ܣ���ɫ��ĩ����Ϻ�ɫ���ú�ɫ��ĩ�Ļ�ѧʽΪ����ͭ����Ӧ�Ļ�ѧ����ʽΪ2NH3+3CuO 3Cu+N2+3H2O��
3Cu+N2+3H2O��
�ʴ�Ϊ��CuO��2NH3+3CuO 3Cu+N2+3H2O��
3Cu+N2+3H2O��
������X��Y��Z���ֶ�����Ԫ�أ����ǵ�ԭ������֮��Ϊ16�����������γ���ɫ���嵥�ʵ�ֻ��H2��N2��O2��ϡ��������⣩�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������˵����NH3��Y��ZΪN2��O2�����ת����ϵ�жϣ�X+Z=B��Z+Y=C��X+Y=A��һ��B�����к��е�Zԭ�Ӹ�����C��������1����˵��ZԪ��ΪH����X��Y��Z�ֱ�ΪO��N��H��A��B��C�ֱ�ΪNO��H2O��NH3���Դ˽����⣮
���������⿼��������ת����ϵ�ķ����жϣ��������ʵ��ۺ�Ӧ�ã���Ҫ����ԭ�ӽṹʾ��ͼ��д����ѧ����ʽ�IJ����жϺ���дԭ��ʵ������ķ���Ӧ�ã�
��1��Ԫ��XΪOԪ�أ�ԭ�ӽṹʾ��ͼΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��C��NH3����X��O2����һ�����������ɻ�����A��NO���Ļ�ѧ����ʽΪ4NH3+5O2
 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2 4NO+6H2O��
4NO+6H2O����3�������£�C��NH3����ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������������ԭ����NH3?H2O
 NH4++OH-���ʴ�Ϊ��NH3?H2O
NH4++OH-���ʴ�Ϊ��NH3?H2O NH4++OH-��
NH4++OH-����4��A��NO����C��NH3����Ӧ����Y��N2����B��H2O���Ļ�ѧ����ʽΪ��4NH3+6NO�T5N2+6H2O���ʴ�Ϊ��4NH3+6NO�T5N2+6H2O��
��5�����������£�Cͨ��װ�к�X�ĺ�ɫ��ĩ��Ӳ�ʲ����ܣ���ɫ��ĩ����Ϻ�ɫ���ú�ɫ��ĩ�Ļ�ѧʽΪ����ͭ����Ӧ�Ļ�ѧ����ʽΪ2NH3+3CuO
 3Cu+N2+3H2O��
3Cu+N2+3H2O���ʴ�Ϊ��CuO��2NH3+3CuO
 3Cu+N2+3H2O��
3Cu+N2+3H2O��������X��Y��Z���ֶ�����Ԫ�أ����ǵ�ԭ������֮��Ϊ16�����������γ���ɫ���嵥�ʵ�ֻ��H2��N2��O2��ϡ��������⣩�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������˵����NH3��Y��ZΪN2��O2�����ת����ϵ�жϣ�X+Z=B��Z+Y=C��X+Y=A��һ��B�����к��е�Zԭ�Ӹ�����C��������1����˵��ZԪ��ΪH����X��Y��Z�ֱ�ΪO��N��H��A��B��C�ֱ�ΪNO��H2O��NH3���Դ˽����⣮
���������⿼��������ת����ϵ�ķ����жϣ��������ʵ��ۺ�Ӧ�ã���Ҫ����ԭ�ӽṹʾ��ͼ��д����ѧ����ʽ�IJ����жϺ���дԭ��ʵ������ķ���Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
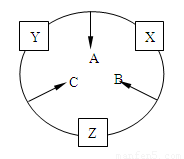
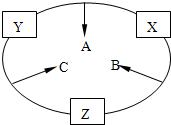 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��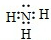
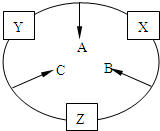 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��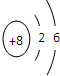
 NH4++OH-
NH4++OH-