��Ŀ����
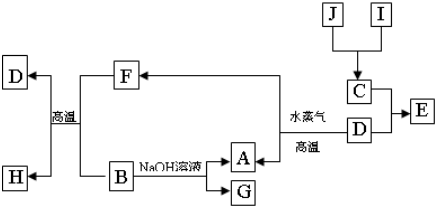
A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ�����֮���ת����ϵ����ͼ��ʾ��

��ش��������⣺
��1��B���Ӧ�Ļ�ѧ����ʽ��______��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����У��Ƿ��ܽ⣿______����ǡ�����
��3����������ˮ�����Һ��������������ӵķ�����______��
��4��д��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽ______��
��5����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L��B��NaOH��Һ��Ӧ�����ӷ���ʽ��______���������B�����ʵ���Ϊ______mol���ú���ĸ�ķ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______mol���������A������Ϊ______g���ú���ĸ�ķ���ʽ��ʾ����
��������õ���Һ�м������������������Һ����ֽ��裬�������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ______��
�⣺A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ����ת����ϵ��֪����Ϊ������������BΪAl��AΪFe��CΪ��������Ϊƫ�����ƣ���Ϊ�Ȼ�����
��1��B���Ӧ�Ļ�ѧ����ʽΪ3Fe3O4+8Al 9Fe+4Al2O3���ʴ�Ϊ��3Fe3O4+8Al
9Fe+4Al2O3���ʴ�Ϊ��3Fe3O4+8Al 9Fe+4Al2O3��
9Fe+4Al2O3��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����з����ۻ���������ȫ�ܽ⣬�ʴ�Ϊ����
��3�����������ӵķ���Ϊȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+���ʴ�Ϊ��ȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+��
��4��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽΪ3Fe+4H2O��g�� Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O��g�� Fe3O4+4H2��
Fe3O4+4H2��
��5����B��NaOH��Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2�����������������ڱ�״����Ϊn L��n��H2��= mol����n��Al��=
mol����n��Al��= mol��
mol��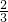 =
= mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2���� ��
��
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L��ת�Ƶ���Ϊ ��2����1-0��=
��2����1-0��= mol���������A������Ϊx���ɵ����غ��֪��
mol���������A������Ϊx���ɵ����غ��֪�� mol��3+
mol��3+ mol��2=
mol��2= ��2����1-0�������x=
��2����1-0�������x= ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��������õ���Һ�м������������������Һ����ֽ��裬�������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�����Ϊ����������������������Al������������Al������������������������������������Ϊ ��100%=30%���ʴ�Ϊ��30%��
��100%=30%���ʴ�Ϊ��30%��
������A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ����ת����ϵ��֪����Ϊ������������BΪAl��AΪFe��CΪ��������Ϊƫ�����ƣ���Ϊ�Ȼ�����Ȼ����Ԫ�ػ�����֪ʶ����ѧ���������
���������⿼��������ƶϣ�ע�����ȷ�Ӧ��DΪ����ɫ����Ϊ������ͻ�ƿڣ����ʵ��ƶ��ǽ����Ĺؼ��������еļ�����Ҫ�漰�����غ㼰��ϵʽ����Ŀ�Ѷ��еȣ�
��1��B���Ӧ�Ļ�ѧ����ʽΪ3Fe3O4+8Al
 9Fe+4Al2O3���ʴ�Ϊ��3Fe3O4+8Al
9Fe+4Al2O3���ʴ�Ϊ��3Fe3O4+8Al 9Fe+4Al2O3��
9Fe+4Al2O3�� ��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����з����ۻ���������ȫ�ܽ⣬�ʴ�Ϊ����
��3�����������ӵķ���Ϊȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+���ʴ�Ϊ��ȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+��
��4��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽΪ3Fe+4H2O��g��
 Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2���ʴ�Ϊ��3Fe+4H2O��g�� Fe3O4+4H2��
Fe3O4+4H2����5����B��NaOH��Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2�����������������ڱ�״����Ϊn L��n��H2��=
 mol����n��Al��=
mol����n��Al��= mol��
mol��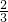 =
= mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2���� ��
������ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L��ת�Ƶ���Ϊ
 ��2����1-0��=
��2����1-0��= mol���������A������Ϊx���ɵ����غ��֪��
mol���������A������Ϊx���ɵ����غ��֪�� mol��3+
mol��3+ mol��2=
mol��2= ��2����1-0�������x=
��2����1-0�������x= ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
����������õ���Һ�м������������������Һ����ֽ��裬�������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�����Ϊ����������������������Al������������Al������������������������������������Ϊ
 ��100%=30%���ʴ�Ϊ��30%��
��100%=30%���ʴ�Ϊ��30%��������A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ����ת����ϵ��֪����Ϊ������������BΪAl��AΪFe��CΪ��������Ϊƫ�����ƣ���Ϊ�Ȼ�����Ȼ����Ԫ�ػ�����֪ʶ����ѧ���������
���������⿼��������ƶϣ�ע�����ȷ�Ӧ��DΪ����ɫ����Ϊ������ͻ�ƿڣ����ʵ��ƶ��ǽ����Ĺؼ��������еļ�����Ҫ�漰�����غ㼰��ϵʽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
a��b��c��d�����ֶ�����Ԫ�أ�a��b��dͬ���ڣ�c��dͬ���壮a��ԭ�ӽṹʾ��ͼΪ ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ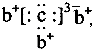 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������
 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ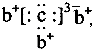 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
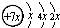 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
 Al��OH��3+OH-
Al��OH��3+OH-