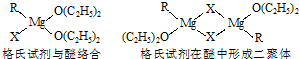
��Ŀ����
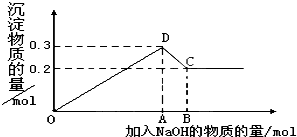 ��MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ�������NaOH�����ʵ�����mol�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����
��MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ�������NaOH�����ʵ�����mol�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ������1��д���������߶η�����Ӧ�����ӷ���ʽ��
OD��
Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3��
Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3��
��DC��
Al��OH��3+OH-�TAlO2-+2H2O
Al��OH��3+OH-�TAlO2-+2H2O
����2��ԭ��Һ��Mg2+��Al3+���ʵ���Ũ��֮��Ϊ
2��1
2��1
����3��ͼ��C���ʾ������
0.8
0.8
mol NaOHʱ��Al3+�Ѿ�ת��ΪAlO2-
AlO2-
Mg2+�Ѿ�ת��ΪMg��OH��2
Mg��OH��2
����4��ͼ���߶�OA��AB=
7��1
7��1
�����������������ӷ�Ӧ��Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O�����ͼ���뷴Ӧ�Ķ�Ӧ��ϵ�����������ʵ�����ԭ���غ������㣮
����⣺��1��OD��þ���Ӻ�������ȫ��ת��Ϊ�����������ӷ���ʽΪMg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����DC��NaOH����ʱ���������ܽ⣬�����ӷ���ʽΪ��Al��OH��3+OH-�TAlO2-+2H2O��
�ʴ�Ϊ��Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O��
��2����ͼ���֪��0��A����Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����A��B����Al��OH��3+OH-�TAlO2-+2H2O��
C���Ӧ�ij���ΪMg��OH��2��D���Ӧ�ij���ΪMg��OH��2��Al��OH��3��
��Mg��OH��2�����ʵ���Ϊ0.2mol��Al��OH��3�����ʵ���Ϊ0.3mol-0.2mol=0.1mol��
��Mg��OH��2��Mg2+��Al��OH��3��Al3+����Һ�������ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
����ԭ��Һ��Mg2+��Al3+���ʵ���Ũ��֮��Ϊ0.2mol��0.1mol=2��1���ʴ�Ϊ��2��1��
��3����Mg2++2OH-�TMg��OH��2��
0.2mol 0.4mol
Al3++3OH-�TAl��OH��3��
0.1mol 0.3mol
Al��OH��3+OH-�TAlO2-+2H2O
0.1mol 0.1mol
��C��NaOH�����ʵ���Ϊ0.4mol+0.3mol+0.1mol=0.8mol����ʱ��������ȫת��ΪAlO2-��þ������ȫת��Ϊ������
�ʴ�Ϊ��0.8��AlO2-��Mg��OH��2��
��4��0��A����Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����
A��B����Al��OH��3+OH-�TAlO2-+2H2O��
���߶�OA��Ӧ��NaOH�����ʵ���Ϊ0.4mol+0.3mol=0.7mol��
�߶�AB��Ӧ��NaOH�����ʵ���Ϊ0.1mol��
�����߶�OA��AB=0.7mol��0.1mol=7��1��
�ʴ�Ϊ��7��1��
�ʴ�Ϊ��Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����Al��OH��3+OH-�TAlO2-+2H2O��
��2����ͼ���֪��0��A����Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����A��B����Al��OH��3+OH-�TAlO2-+2H2O��
C���Ӧ�ij���ΪMg��OH��2��D���Ӧ�ij���ΪMg��OH��2��Al��OH��3��
��Mg��OH��2�����ʵ���Ϊ0.2mol��Al��OH��3�����ʵ���Ϊ0.3mol-0.2mol=0.1mol��
��Mg��OH��2��Mg2+��Al��OH��3��Al3+����Һ�������ͬ��Ũ��֮�ȵ������ʵ���֮�ȣ�
����ԭ��Һ��Mg2+��Al3+���ʵ���Ũ��֮��Ϊ0.2mol��0.1mol=2��1���ʴ�Ϊ��2��1��
��3����Mg2++2OH-�TMg��OH��2��
0.2mol 0.4mol
Al3++3OH-�TAl��OH��3��
0.1mol 0.3mol
Al��OH��3+OH-�TAlO2-+2H2O
0.1mol 0.1mol
��C��NaOH�����ʵ���Ϊ0.4mol+0.3mol+0.1mol=0.8mol����ʱ��������ȫת��ΪAlO2-��þ������ȫת��Ϊ������
�ʴ�Ϊ��0.8��AlO2-��Mg��OH��2��
��4��0��A����Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����
A��B����Al��OH��3+OH-�TAlO2-+2H2O��
���߶�OA��Ӧ��NaOH�����ʵ���Ϊ0.4mol+0.3mol=0.7mol��
�߶�AB��Ӧ��NaOH�����ʵ���Ϊ0.1mol��
�����߶�OA��AB=0.7mol��0.1mol=7��1��
�ʴ�Ϊ��7��1��
���������⿼��ѧ���������ӷ�Ӧ������������Ļ���������������㣬��ȷͼ����ÿ�η����Ļ�ѧ��Ӧ�ǽ����Ĺؼ�����ע������ԭ���غ�ķ��������

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ