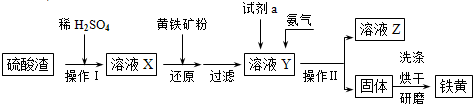
��Ŀ����
ѡ��ʹ����ͼ������(��ҩƷ)����֤��ͭ������Ũ���ᷴӦ�����������к���NO(N2��O2�����������ɿ���)����֪����NO+NO2+2OH-====2NO-2+H2O��������Һ���¶ȣ�NO2��21 �棻NO��-152 �档
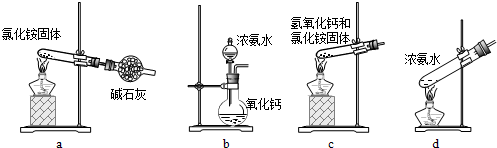
(1)���мס��ҡ�����λͬѧ�ֱ��������������ʵ�鷽��(����������˳���ʾ)��
�ף�A��C��F��D��B
�ң�A��B��C��E
����A��E��D��B
�ס��ҡ�����λͬѧ�����ʵ�鷽���Ƿ�����֤Cu������ŨHNO3��Ӧ�����������к���NO��
�ף�_________���ң�_________������_________��(��ܡ����ܡ�)
����˵��������֤����Ҫԭ��(������ʵ�鷽��������֤�����С�ⲻ�ûش�)��
������___________________________________________��
�ҷ�����___________________________________________��
��������___________________________________________��
(2)��Ӧǰ��ͨ��������N2����Ŀ����_______________________________________��
(3)ȷ�������к���NO�������ǣ�__________________________________________��
(4)���O2��������װ��B�з�����Ӧ�����ӷ���ʽ�ǣ�__________________________��
(1)�� ���� ����
�ҷ������������ͨ��Bʱ��������Ӧ��NO+NO2+2NaOH====2NaNO2+H2O��NO�����ģ�����ʵ������֤�Ƿ���NO
���������������ͨ��Eʱ��������Ӧ��3NO2+H2O====2HNO3+NO��ʹ����NO2ת��ΪNO���������ţ��Ӷ�����ȷ��Cu��ŨHNO3��Ӧ�Ƿ���NO����
(2)�ž�����װ���еĿ�������ֹ��Ӧ������NO��������O2����
(3)װ��D��ͨ��O2���к���ɫ��������
(4)4NO2+O2+4OH-====4![]() +2H2O
+2H2O
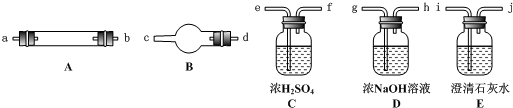
�����������ʵ��Ŀ������֤ͭ������Ũ���ᷴӦ�����������к���NO���ʷ���ʱӦ�Դ�Ϊ������չ��˼·������ͭ��Ũ���ᷴӦ����Ҫ������NO2����������NO��ˮ������������֤��ǰ���Ȱ�NO2��ˮ��������������������Ϣ������Һ���¶ȣ�NO2��21 �棻NO��-152 �棩��������ͼ��װ�ÿ�֪��Ӧ��Fװ��(��ˮԡ)������NO2��NO������F��NO2����ΪҺ��������U�ι��У���NO�ݳ�����Ȼ���ڷ���NO2��NO֮ǰ�������Ȱѻ�������е�ˮ������Cװ�ó�����������ͨ����ˮԡʱ��ˮ����������ˮ��NO2��Ӧ��3NO2+H2O====2HNO3+NO�����ڲ�������NO����Ӱ���ʵ�������ж����������Aװ���в��������Ⱦ���C�ٵ�F��Ҫ��֤��F������������NO�������NO������ѡ��Ӧ�������Ե�װ�ã���Dװ�á�NO��ͨ��D�е�O2��Ӧ(2NO+O2====2NO2)�к���ɫ���֣�����֤������NO��NO2������Ⱦ���������������������װ���Գ�ȥ��Dװ���г�����NO��NO2������������֪��Ϣ�ٿ�֪Ӧ�ü�Һ���գ���Bװ�á�
���⣬�ڷ�Ӧ����ǰ�������Aװ���еĿ����Ÿɾ����Է�������NO�������е�O2������
�������������������ȷ���ҡ�������
��O2����ʱ��D�г���������ΪNO2������O2�Ļ�������ͨ��Bװ����ʱ����ȫ��ת��Ϊ������(��4NO2+O2+2H2O====4HNO3)��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�