��Ŀ����
��18�֣���ѧ̽�������ȷ��������̽������о������Ŀ�ѧ�ԣ��о����̵ļƻ��ԡ��о�Ŀ����ȷ�ԡ�
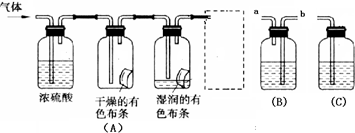
��һ��ѧ�����ף�Ӧ����ͼװ�ã�A����ʾ��ķ����о����������ʣ������������Ҫ�ɷ������������п�����ˮ����������ش��������⣺
��1�������о�(ʵ��)����ҪĿ����
��2��Ũ����������� �����о�Ŀ��ֱ����ص�ʵ�������� ________________ _ ��
��3������е�װ��Ӧѡ�� ���B����C��������ʢ�ŵ��Լ�
Ϊ �����Լ����ƣ� ��ʹ�ø�װ�õ�Ŀ���� ����װ���ڷ�����Ӧ�Ļ�ѧ����ʽΪ ��
������ѧ�����ң����ʵ��̽����������������Ĥ�����ʣ�����Ƭ��������Ĥ��Ͷ��Ũ�Ȼ�ͭ��Һ�У�������ܿ����һ�㺣��״����ɫ���ʡ�����ͬ������ƬͶ��ͬŨ�ȵ�����ͭ��Һ�У��ڶ�ʱ������Ƭ�����Ա仯���ش��������⣺
��1����Ƭ������ֵİ���ɫ���ʵĹ����з��������ӷ�Ӧ����ʽ�� ��
��2��ͬѧ���ң���Ϊ�������Ȼ�ͭ��Һ��Ѹ�ٷ�Ӧ������ͬŨ�ȵ�����ͭ��Һ�ڶ�ʱ���ڲ���Ӧ��ԭ���ǡ����������ƻ����������污Ĥ,����������Ӳ��ܡ������������ʵ�鷽��������֤�����������˼·������пո�
[ʵ�鷽��] ������ͭ��Һ�м�����Ƭ�������������ټ��� �����Լ����ƣ�������Ӧ���Լӿ��ˣ�˵�������ƶ���ȷ��
��18�֣���һ��
��1���Ƚϸ���������ͳ�ʪ��������Ư���ԣ����о�������Ư����ʵ��Ⱥ����𰸾��ɣ���2�֣�
��2�����������е�ˮ�� �������ɫ��������ɫ��ʪ�����ɫ������ɫ�������𰸾��ɣ�
������2�֣���4�֣�
��3��B ��NaOH��Һ����ֹ�ж���Cl2��Ⱦ������2NaOH + Cl2== NaCl + NaClO+ H2O ������2�֣���8�֣�
��������1��2Al+3Cu2+��3Cu+2Al3+;��2�֣�
��2���Ȼ��ƣ������������2�֣�
����:��