��Ŀ����
(11��)ij��ѧС�����ʵ��̽��̼���ơ�̼�����Ƶ����ʣ�ʵ�����£�ȡ��֧�Թֱܷ����10 mL��ͬŨ�ȵ�ϡ���ᣬ��������װ��0 5 g��Na2CO3��NaHCO3��ĩ��С����ֱ����������Թ��ϣ��������ڵĹ����ĩͬʱ�����Թ��У���֪�����������۲�ʵ������
(l)��֧�Թ��о��������壬���в�������Ͽ��Ϊ________(��Թ�I�����Թ�II��)��Ͷ��________���ѧʽ�����Թ��������ñȽϴ�
��2����ͬѧ�����������Թܣ������Թ�I���䣬�Թܢ���ȣ��ɴ����������״̬��Σ�NaHCO3��HCl��ӦΪ���ȷ�Ӧ��Na2CO3��HCl��ӦΪ���ȷ�Ӧ��
Ϊ��һ��̽��Na2CO3��NaHCO3�����ᷴӦ�������仯����ͬѧ��������ʵ�飬��������Ϊ�������Լ�1�м����Լ�2�����衢�ⶨ�¶ȣ��ھ��á��ⶨ�¶ȣ����ټ����Լ�3�����衢�ⶨ�¶ȡ���¼���õ��������ݣ�
����ʵ����Ҫ�õ��IJ���������________��
��ͬѧ�ɵó����ۣ�
��NaHCO3���ܽ����________������ȡ����ȡ�����ͬ����Na2CO3���ܽ����________��
��CO32����H����ӦΪһ��Ӧ������ȡ������ȡ�����ͬ����HCO3����H����ӦΪ________��Ӧ��
��3���Ƚϼ���ͬѧ��ʵ�飬����Ϊ ________����ס����ҡ�����ʵ�鷽���������������ܡ�
��1���Թ�I��NaHCO3��
��2���ձ������������¶ȼƣ������ȡ����ȣ��ڷ��ȡ����ȣ�
��3����
���������������1��̼��������̼�������Ũ�ȵ����ᷴӦ��̼�����Ƶķ�Ӧ���ʿ죬��Ϊ̼������ֻ����1�������ӾͿ����ɶ�����̼���壬���Բ�������Ͽ�����Թ�I����������̼��������̼�������������ᷴӦ��̼�����Ʋ���������࣬����Ͷ��NaHCO3���Թ��������ñȽϴ�
��2������ʵ������жϣ���Ҫ�IJ����������ձ������������¶ȼƣ�
�ٴӱ������ݿ�֪��ʱ������19��0ºC��̼�������ܽ���¶ȵ���19��0 ºC��˵��̼�����Ƶ��ܽ�������ȣ�ͬ��̼���Ƶ��ܽ�����Ƿ��ȣ�
��̼����������ķ�Ӧ��2����CO32��+H��=HCO3����HCO3��+ H��=H2O+CO2������̼�����Ƶķ�Ӧ�¶��ж�HCO3����H����ӦΪ���ȷ�Ӧ����̼����������ķ�Ӧ�Ƿ��ȷ�Ӧ������̼���������ᷴӦ�ĵ�һ���Ƿ��ȷ�Ӧ��
��3����ͬѧ��ʵ�鷽������������ͬѧ���жϿ�������Ϊ�ܽ���̵���ЧӦ��ɵ��¶ȵIJ��죬����˵���Ƿ�Ӧ����ЧӦ��ɵģ���ͬѧͨ���ȽϷ�Ӧ����¶ȣ��жϷ�Ӧ����ЧӦ�������ȸ���˵������
���㣺����̼�����ơ�̼���Ƶ�����ʵ�飬��ʵ��ķ����ж�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ʵ���������ʵ��Ԥ��ʵ��Ŀ�Ļ����ý���һ�µ���( )
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ij��Һ ð������ ð������ | ˵��ԭ��Һ��һ������CO32- |
| B | SiO2�봿����¿�����CO2 | ˵����������Ա�̼��ǿ |
| C | �⻯����Һ�����Ի�ɫ | ������I-����ԭ��������I2������Һ�� |
| D | ��������Ũ�����н��ݺ���������ˮ��ϴ��Ȼ�����CuSO4��Һ�в���Ӧ | ˵�����������γ���һ�������ȶ�������Ĥ |
����16�֣���ѧʵ���ǿ�ѧ̽���Ļ�������ش��й�ʵ�����⣺
��1��������ĸ�ʵ��װ������������������ȱ�ݣ�������ȫ��ȷ���� ��
��2��Ҫ��������Bװ�ð����Ѽ����IJ����� ���Թ��Ѽ�����
��3��ClO2��һ�ְ�ȫ����Ч�����ס�ǿ��ɱ���������������ұ���
�����±����Կ���������Һ̬ClO2���������ܷ⡢ ��ClO2��Ӧ�����ӷ���ʽ ���۲��¡�ͼA����Ҫ��NaClO2��Һ�Ƶò����ᾧˮ�ľ��壬�����������ᾧ������������ ��Ӧ��������������¶ȷ�Χ�� ��
| ɫ̬ | ���ڼ� | ����1Kpa�����Ȼ����� | |
| �������� | -59-11�� ���ɫҺ�� | �����������κ������� | ��ը |
�ڹ�ҵ�ó�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼB��ʾװ��ģ�ҵ�Ʒ���ȡ���ռ�Һ̬ClO2����ģ����ͼB��װ��ͼ��ͼ�����ڴ������Ӧͼ�в�������װ��ͼ��������Ҫ�ı�ע��

��15�֣�S2Cl2�����������л����Ȼ����Լ���ʵ���ҿ���������װ�����Ʊ�S2Cl2�������ּг���������ȥ��
��֪�����Ʊ��ķ�Ӧ����ʽΪ��
�ڷ�Ӧ�漰�ļ��������������£�
| ���� ���� | �۵� | �е� | �Ż�� | �������� |
| ��� | 119.2�� | 446.6�� | 363�� | / |
| ��� | 112.8�� | 446.6�� | 363�� | / |
| S2Cl2 | -77�� | 137�� | / | ��ˮǿ�ҷֽ�����S��SO2��HCl |
��1��A��������װ��ʱ���ź�����̨֮��Ӧ�ȹ̶� �����������ƣ�������װ��װ����Ϻ�Ӧ�Ƚ��� ���������Լ�������ˮ��ˮ���� ���a����b������ʵ����ϣ�A�в��ٲ�������ʱ���ɲ��װ�ã����ʱ�����Ƚ��еIJ���Ӧ�� ��
��2��S2Cl2�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��д�������ʽ ���û�ѧ����ʽ��ʾ��ȥCװ�õĺ�� ����ֱ�Ӽ��ȴ���ˮԡ���ȵĺ���� ��
��3����б��б����Ϊ �����۵㡢�е㡢�Ż�����Ϣ�õ�����ʾ�� ��
��4��M��Һ����ѡ�������Լ��е� ������ţ�
��̼������Һ ���Ȼ�����Һ ������������Һ �ܸ��������Һ
�����г����⣬����������Ԫ�أ���̼Ԫ�غ���Ԫ�ء�����̼��Ҫ��̼��������̬���ڣ���ʹ�������ܼ�Ӳ���࣬������������;����һ���������ֵ�ԭ�ϡ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ�ã��ⶨ�����еĺ�̼����
A B C D E
��ش��������⣺
��1���������������к�Ԫ�أ���ʹ���������ȴ��ԡ���Ԫ�������������п��ܴ��ڵļ�̬��
| A����2���� | B��0�� �� | C��+4���� | D��+6 |
��3��D��30% ˫��ˮ�������� ������װ�ã����ⶨ�ĺ�̼���� ���ƫ�ߡ�����ƫ�͡���Ӱ�족��
��4����Ӧ��ɺ�����֤����������Ԫ�أ�������Ƶ�ʵ�鷽���ǣ�д��ʵ�鲽�衢���� ��
��5����C�ܵ���Ʒ��ַ�Ӧ���E�����ɵij���Ϊbg�������������еĺ�̼��Ϊ ��
��6��ʵ������У�����ȤС��Ӧע��İ�ȫ������ ������дһ�֣�
Ϊ�ⶨ��������茶��塾��NH4��2Fe (SO4)2 �� xH2O�������ĺ�����ijʵ��С����������ʵ�飺
����һ���õ�����ƽȷ����5.000g��������茶��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ���Fe2+ǡ��ȫ��������Fe3+��ͬʱ��MnO4-����ԭ��Mn2+��
���ظ���������Ρ�
��ش��������⣺
��1�����������������Һ�IJ������������ǣ������� ��ת�ơ�ϴ�Ӳ�ת�ơ� ��ҡ�ȡ�
��2���� �ζ���ʢ��KMnO4��Һ��
��3�����������һ��KMnO4��Һ,���� ,������ζ��յ㡣��Ӧ�����ӷ���ʽ��
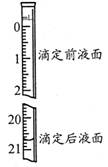
��4���ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
ʵ���øþ�����������������Ϊ ����������λС����
��5�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ���������Ʒ���������������� �����ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�δ�����ζ�ʱ��ȥKMnO4����Һ�������
���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲� ��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ����Ӷ����������Ʒ���������������� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����