��Ŀ����
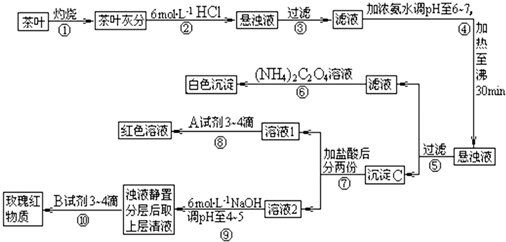
�����ҹ�����ϲ������Ʒ��ijУ��ѧ��ȤС���ͬѧ�������ʵ�������Լ����Ҷ�к��иơ����������ֽ���Ԫ�ء����IJ������ϣ������[(NH4)2C2O4]������ˮ�������(CaC2O4)������ˮ��Ca2+��Al3+��
Fe3+��ȫ������pH��Ca(OH)2 pH��l3��Al(OH)3 10��pH��5��Fe(OH)3 pH��3.7���Ը����������̼���Ϣ���
Fe3+��ȫ������pH��Ca(OH)2 pH��l3��Al(OH)3 10��pH��5��Fe(OH)3 pH��3.7���Ը����������̼���Ϣ���

�Ը����������̼���Ϣ��գ�
(1)����۲����У���Ҫ�õ�����̨����Ȧ�����С��ձ���__________��__________��������
(2)����ڼ������������________________________��
(3)����ܵ�Ŀ����________________________��
(4)д������C������Ҫ���ʵĻ�ѧʽ��____________��
(5)д����������Ӧ�����ӷ���ʽ��________________��
(6)д���ᷢ����Ӧ�����ӷ���ʽ��________________��
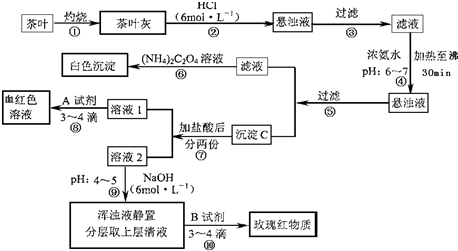
(1)����۲����У���Ҫ�õ�����̨����Ȧ�����С��ձ���__________��__________��������
(2)����ڼ������������________________________��
(3)����ܵ�Ŀ����________________________��
(4)д������C������Ҫ���ʵĻ�ѧʽ��____________��
(5)д����������Ӧ�����ӷ���ʽ��________________��
(6)д���ᷢ����Ӧ�����ӷ���ʽ��________________��
(1)©����������
(2)ʹ��Ҷ���еĸơ������������ܻ�����ת���ɿ������Ȼ���
(3)ʹ����������������������ʽ��ȫ����
(4)Fe(OH)3��Al(OH)3
(5)Fe3++3SCN- Fe(SCN)3
Fe(SCN)3
(6)Fe3++3OH-=Fe(OH)3��
(2)ʹ��Ҷ���еĸơ������������ܻ�����ת���ɿ������Ȼ���
(3)ʹ����������������������ʽ��ȫ����
(4)Fe(OH)3��Al(OH)3
(5)Fe3++3SCN-
 Fe(SCN)3
Fe(SCN)3 (6)Fe3++3OH-=Fe(OH)3��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ