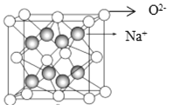
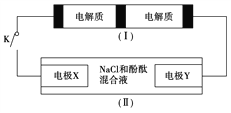
��Ŀ����
����Ŀ���������������Ļ��Գɷ֣����ڿ�ǻ�в������ոУ��ܹ���Ѫѹ�͵��̴��Ĺ�Ч�������ںܴ�̶���Ԥ�����ಡ��Ҳ�ܻ��⼡��ؽ���ʹ����������������ͨʽ���Ա�ʾΪ![]() (RΪ����)������һ���������������J�ĺϳ�·����ͼ��ʾ��
(RΪ����)������һ���������������J�ĺϳ�·����ͼ��ʾ��
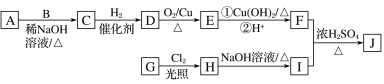
��֪����A��B��E��Ϊͬϵ�����A�ķ���ʽΪC5H10O��B����Է�������Ϊ44��A��B�ĺ˴Ź���������ʾ��������塣
��J�ķ���ʽΪC15H22O4��
��R1CHO��R2CH2CHO![]()
![]() ��
��
�ش��������⣺
(1)G�����������ŵ�����Ϊ__________��
(2)��A��B����C�Ļ�ѧ����ʽΪ__________��
(3)��C����D�ķ�Ӧ����Ϊ________��D�Ļ�ѧ����Ϊ_________��
(4)��H����I�Ļ�ѧ����ʽΪ________��
(5)��G��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵĹ���_____��(���������칹)�����к˴Ź���������ʾ��������_______(д�ṹ��ʽ)��
���𰸡��Ѽ���(��)�ǻ� (2)(CH3)3CCHO��CH3CHO![]()
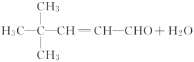 �ӳɷ�Ӧ(��ԭ��Ӧ) 4,4-����-1-�촼
�ӳɷ�Ӧ(��ԭ��Ӧ) 4,4-����-1-�촼 ![]() ��2NaOH
��2NaOH![]()
![]() ��NaCl��H2O 8
��NaCl��H2O 8 ![]()
��������
������J�ķ���ʽΪC15H22O4�����J�ĽṹΪ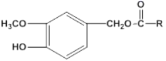 ����RΪ��C6H13����˽������ͼ֪��IΪ
����RΪ��C6H13����˽������ͼ֪��IΪ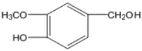 ��FΪC6H13COOH�����GΪ
��FΪC6H13COOH�����GΪ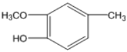 ��HΪ
��HΪ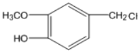 �������֪��Ϣ��B����Է�������Ϊ44��B�˴Ź���������ʾ������壬��BΪCH3CHO��A��BΪͬϵ���AΪC4H9CHO��A�ĺ˴Ź���������ʾ������壬��AΪ(CH3)3CCHO��CΪ(CH3)3CCH=CHCHO��DΪ(CH3)3CCH2CH2CH2OH��EΪ(CH3)3CCH2CH2CHO��FΪ(CH3)3CCH2CH2COOH���ݴ˷�������
�������֪��Ϣ��B����Է�������Ϊ44��B�˴Ź���������ʾ������壬��BΪCH3CHO��A��BΪͬϵ���AΪC4H9CHO��A�ĺ˴Ź���������ʾ������壬��AΪ(CH3)3CCHO��CΪ(CH3)3CCH=CHCHO��DΪ(CH3)3CCH2CH2CH2OH��EΪ(CH3)3CCH2CH2CHO��FΪ(CH3)3CCH2CH2COOH���ݴ˷�������
��1��GΪ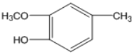 �����������ŵ����Ѽ���(��)�ǻ����ʴ�Ϊ���Ѽ���(��)�ǻ���
�����������ŵ����Ѽ���(��)�ǻ����ʴ�Ϊ���Ѽ���(��)�ǻ���
��2��AΪ(CH3)3CCHO��BΪCH3CHO��A��B����C�Ļ�ѧ����ʽΪ(CH3)3CCHO��CH3CHO![]()
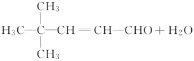 ��
��
��3��CΪ(CH3)3CCH =CHCHO��DΪ(CH3)3CCH2CH2CH2OH��C�����������ӳɷ�Ӧ����D��DΪ(CH3)3CCH2CH2CH2OH������Ϊ4��4-����-1-�촼���ʴ�Ϊ���ӳ�(��ԭ)��4��4-����-1-�촼��
��4��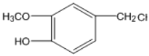 ������������Һ�з���ˮ�ⷴӦ����I����ѧ����ʽΪ
������������Һ�з���ˮ�ⷴӦ����I����ѧ����ʽΪ![]() ��2NaOH
��2NaOH![]()
![]() ��NaCl��H2O���ʴ�Ϊ��
��NaCl��H2O���ʴ�Ϊ��![]() ��2NaOH
��2NaOH![]()
![]() ��NaCl��H2O��
��NaCl��H2O��
��5��GΪ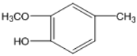 ��G��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵ���
��G��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵ���![]() ��
��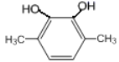 ��
��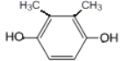 ��
��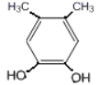 ��
��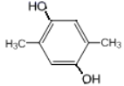 ��
��![]() ��
��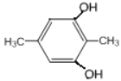 ��
��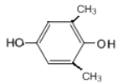 ��8�֣����к˴Ź���������ʾ2������
��8�֣����к˴Ź���������ʾ2������![]() ���ʴ�Ϊ��8��
���ʴ�Ϊ��8��![]() ��
��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�