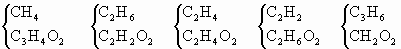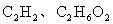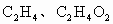��Ŀ����
AΪ����BΪ���ĺ���������ɵ����ʵ�����A��B��ɵĻ����0.05mol��0.125mol����������ȫȼ�գ�����0.1mol![]() ��0.1mol
��0.1mol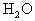 ����ͨ������ش�
����ͨ������ش�
(1)�ӷ���ʽ�ĽǶȿ����û����������ļ��ֿ���________
(2)��ȡһ������A��B��ȫȼ�գ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ����
����������һ������A��B�ķ���ʽ�ֱ���________��________��
�������ɵ� �����ʵ���һ������A��B�ķ���ʽ�ֱ���________��________��
�����ʵ���һ������A��B�ķ���ʽ�ֱ���________��________��
(3)��ȡamol������Ȼ�ϵ�A��B�Ļ����ڹ�����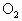 ����ȫȼ�գ�
����ȫȼ�գ�
����������Ϊ��ֵ�����ֵΪ________mol(�ú�a�Ĵ���ʽ��ʾ����ͬ)��
����������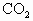 ����Ϊ��ֵ����������ˮ��������Χ________��
����Ϊ��ֵ����������ˮ��������Χ________��
�𰸣�
������
������
|
(1) (2)�� �� (3)��2.5a ��18a��54a |

��ϰ��ϵ�д�
�����Ŀ