��Ŀ����
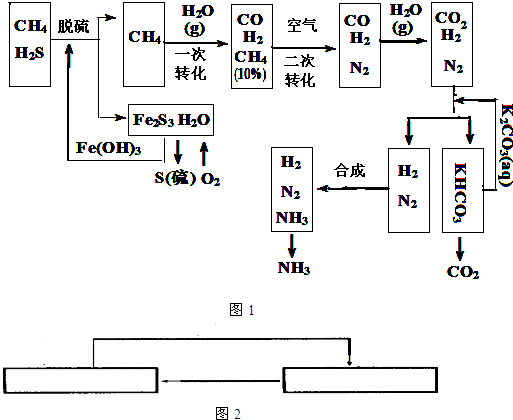
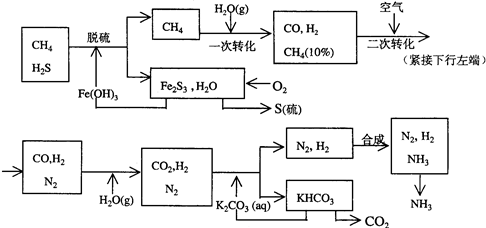
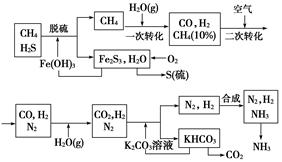
������Ȼ���ϳɰ��Ĺ�������ʾ��ͼ����

�����������̣����������գ�
��1����Ȼ������ʱ�Ļ�ѧ����ʽ��_____________��
��2��ͼ��CH4�ĵ�һ��ת�������еĻ�ѧ����ʽ��_______________��
��3����������������ѭ����һ��K2CO3(aq)ѭ��������N2��H2ѭ��������_______���ѧʽ��ѭ����
��4��K2CO3��aq���� CO2��Ӧ�ڼ�ѹ�½��У���ѹ������������_______����ѡ�۷֣���
a.����ԭ��
b.��������ԭ��
c.����к�ԭ��
��1����Ȼ������ʱ�Ļ�ѧ����ʽ��_____________��
��2��ͼ��CH4�ĵ�һ��ת�������еĻ�ѧ����ʽ��_______________��
��3����������������ѭ����һ��K2CO3(aq)ѭ��������N2��H2ѭ��������_______���ѧʽ��ѭ����
��4��K2CO3��aq���� CO2��Ӧ�ڼ�ѹ�½��У���ѹ������������_______����ѡ�۷֣���
a.����ԭ��
b.��������ԭ��
c.����к�ԭ��
��1��3H2S��2Fe(OH)3=== Fe2S3��6H2O
��2��CH4+H2O CO+3H2
CO+3H2
��3��Fe(OH)3
��4��b
��2��CH4+H2O
 CO+3H2
CO+3H2��3��Fe(OH)3
��4��b

��ϰ��ϵ�д�
�����Ŀ