��Ŀ����
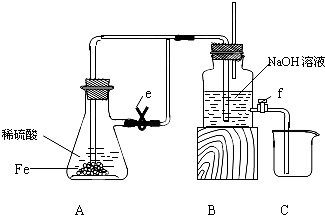
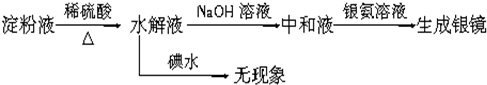
ij����С�����������ʵ�鷽����֤Ag��ŨHNO3��Ӧ�Ĺ����п��ܲ���NO����ʵ������ͼ����

(1)�ⶨ��������ʵ���
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol/L��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ________mol��
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol/L��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ________mol��

(2)�ⶨNO�����
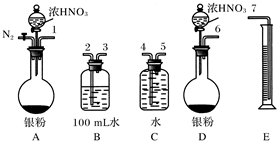
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������___________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����________(������ܿڱ��)��
���ٲⶨNO�������
(3)����ɷַ���
��ʵ����NO�����Ϊ112.0 mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO��������д�������жϵļ�����̡�
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������___________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����________(������ܿڱ��)��
���ٲⶨNO�������
(3)����ɷַ���
��ʵ����NO�����Ϊ112.0 mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO��������д�������жϵļ�����̡�
(1)0.008mol
(2)��A����ΪAװ�ÿ���ͨ��N2��װ���еĿ����ž�����ֹNO�������е�������������123547
(3) �У������жϵļ�����̣���ΪNO2��ˮ��Ӧ����NO�������0.004mol��С���ռ�����NO�������0.005mol��
(2)��A����ΪAװ�ÿ���ͨ��N2��װ���еĿ����ž�����ֹNO�������е�������������123547
(3) �У������жϵļ�����̣���ΪNO2��ˮ��Ӧ����NO�������0.004mol��С���ռ�����NO�������0.005mol��

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
�����Ŀ


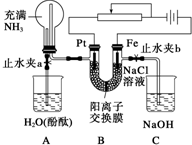 ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������