��Ŀ����
25��ʱ����25 mL0.1 mol��L-1��NaOH��Һ�У���μ���0.2 mol��L-1��CH3COOH��Һ����Һ��pH����������ϵ��ͼ�����з�����ȷ����
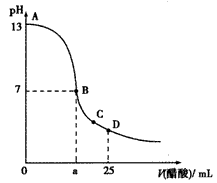
[ ]
A��B������a=12.5
B��C��ʱ��Һ���У� c(Na+)>c(CH3COO-)>c(H+)>c(OH-)
C��D��ʱ��Һ���У� c( CH3COO-)+c(CH3COOH)=2c(Na+)
D��������A��B�������һ�㣬��Һ�ж��У�c(CH3COO-)>c(Na+)��c(OH-)>c(H+)
B��C��ʱ��Һ���У� c(Na+)>c(CH3COO-)>c(H+)>c(OH-)
C��D��ʱ��Һ���У� c( CH3COO-)+c(CH3COOH)=2c(Na+)
D��������A��B�������һ�㣬��Һ�ж��У�c(CH3COO-)>c(Na+)��c(OH-)>c(H+)
C

��ϰ��ϵ�д�
�����Ŀ
 ˮ�ĵ���ƽ��������ͼ��ʾ��?
ˮ�ĵ���ƽ��������ͼ��ʾ��? M(OH)2
M(OH)2 2H+��MO22��
2H+��MO22�� M(OH)2
M(OH)2 2H+��MO22��
2H+��MO22��